DelMar Pharmaceuticals' Promising New Data Support the Potential of VAL-083 to Meet Significant Unmet Medical Needs in the Treatment of Glioblastoma Multiforme
Data Presented at the 19th Annual Society for Neuro-Oncology Meeting Demonstrate VAL-083 Superiority to Temozolomide in Key Preclinical Models
DelMar Continues to Elevate Dose of VAL-083 to New Levels in Clinical Trials
VANCOUVER, British Columbia, MENLO PARK, Calif., and MIAMI, Nov. 17, 2014 /PRNewswire/ -- DelMar Pharmaceuticals, Inc. (OTCQB: DMPI) ("DelMar" and "DelMar Pharma") today announced the presentation of new data supporting the development of VAL-083 (dianhydrogalactitol), which is currently undergoing clinical development in the United States as a potential new chemotherapy for the treatment of refractory glioblastoma multiforme (GBM), the most common and deadly form of human brain cancer.
The data were presented in two additional abstracts on Friday November 14, 2014 during the evening scientific session at the Society for NeuroOncology Annual Meeting. Overall, DelMar presented three abstracts at the meeting.
On Friday, DelMar announced the presentation of an abstract entitled In vivo efficacy of VAL-083 in the treatment of MGMT-positive glioblastoma multiforme (GBM), which highlighted the company's research into the activity of VAL-083 in comparison to standard-of-care temozolomide in animal models of GBM.
The presentation can be viewed at: DELMAR INVIVO DATA PRESENTATION SNO2014
The second abstract entitled VAL-083 is a novel N7 alkylating agent that inhibits the growth of glioma stem and non-stem cultures, including temozolomide-resistant lines presented by DelMar's collaborators from the University of California San Francisco (UCSF), provided further pre-clinical validation of VAL-083's different and potentially superior activity compared to standard-of-care temozolomide.
The presentation can be viewed at: DELMAR INVITRO DATA PRESENTATION SNO2014
The third abstract entitled Phase I/II study of dianhydrogalactitol (VAL-083) in patients with recurrent malignant glioblastoma multiforme (GBM) provided an update on DelMar's ongoing Phase I/II clinical trial with VAL-083 in the treatment of refractory glioblastoma.
The presentation can be viewed at: DELMAR CLINCAL PRESENTATION SNO2014
Overview of UCSF in vitro Research Findings
"Researchers from UCSF previously demonstrated that the response of both cancer stem cells (CSC) and paired non-CSC cultures isolated from glioblastoma patients to temozolomide, the current standard of care in the treatment of glioblastoma was dependent on the presence or absence of an enzyme called O6-methylguanine methyltransferase (MGMT)," said Jeffrey Bacha, president & CEO of DelMar Pharmaceuticals. "We collaborated with UCSF to investigate how the same cultures respond to VAL-083 alone or in combination with radiation, and how the response would compare to temozolomide."
GBM is the most common and deadly form of human brain cancer. The standard of care for newly diagnosed GBM patients is surgical resection followed by temozolomide and radiation and subsequent maintenance therapy with temozolomide alone. Temozolomide is effective for a minority of patients that low expression of MGMT, a DNA repair enzyme that repairs the cytotoxic damage temozolomide causes to cancer cells. The majority of patients exhibit high expression of MGMT and are resistant to temozolomide.
"VAL-083 is a first-in-class alkylating agent that crosses the blood brain barrier and is currently in clinical trials for glioma patients with recurrent GBM. We hypothesized that the N7 alkylating agent, VAL-083, is not subject to MGMT mediated repair and might therefore be a more potent chemotherapeutic for the treatment of GBM," said Joseph Costello, Professor of Neurological Surgery at UCSF.
Patient derived GBM cells were treated with increasing doses of temozolomide or VAL-083 and cell cycle analysis was performed four days after treatment. VAL-083 proved to be effective against temozolomide resistant cultures, even at single micromolar (uM) doses.
The activity of VAL-083 and temozolomide were compared by treating CSCs with a 5uM dose of VAL-083 or a 50uM dose of temozolomide in combination with a standard 2 gray (Gy) dose of radiation. CSCs were examined for cell viability four (4) days post treatment. The cultures studied were not sensitive to the combination of temozolomide and radiation but were sensitive to treatment with the combination of VAL-083 and radiation. Further, VAL-083 was shown to increase cancer cells sensitivity to radiation at doses below 2uM.
In summary, UCSF researchers demonstrated that:
- VAL-083 appears to cause cell cycle arrest and loss of cell viability at lower concentrations than temozolomide;
- Unlike temozolomide, VAL-083 is active against stem and non-stem cells and its activity affected by MGMT status. All cultures tested were sensitive to VAL-083 exposure; and
- For all cultures tested, a potential additive effect of VAL-083 with radiation therapy was observed, particularly at low concentrations of VAL-083.
"These in vitro results suggest that VAL-083 may provide greater killing of MGMT unmethylated tumor cells compared to the standard-of-care chemotherapy," added Prof. Costello.
Mr. Bacha added, "While this program is progressing in clinical trials for GBM patients having failed approved therapies we continue believe in the importance of nonclinical research to differentiate VAL-083 not only for continued validation of its promise as a potential treatment for refractory GBM, but also to support its potential as an alternative front-line chemotherapy in the future."
Overview of Clinical Data Presentation:
To date, 13 male and 10 female subjects with GBM have completed safety analysis at doses up to 40mg/m2 in DelMar's VAL-083 clinical trial without encountering dose limiting toxicity (DLT). Seven additional subjects with CNS metastases were enrolled at lower doses; however, enrollment at doses above 5mg/m2 has been limited to GBM.
Historical studies sponsored by the US National Cancer Institutes (NCI) achieved promising results in newly diagnosed and recurrent GBM using a dosing regimen of 25 mg/m2/day for five days every five weeks. Historically, dose limiting hematologic toxic effects were noted on white blood cell (WBC) and platelet counts. For example, Egan etal. (1979) observed nadir of 2,100/mL (lymphopenia) and 88,000/mL (thrombocytopenia), respectively. In a separate study, a dose of 40mg/m2/day for five days resulted in a median platelet nadir of 31,000/mL and WBC nadir of 2,300/mL. In general, nadir occurred within three weeks and returned to normal within seven (7) days. Anemia, nausea and vomiting were usually mild to moderate. No renal, hepatic, central nervous system, cardiac, or pulmonary toxicity was identified.
The DelMar dosing regimen uses a cycle of treatment on the first three days of every three weeks. "We hypothesized that this regimen along with improvement in management of myelosuppression in the modern era would allow for more aggressive dosing and potentially improved patient outcomes," said Mr. Bacha.
A summary of recent, ongoing and planned dose cohorts compared to the historical regimen used in NCI-sponsored Phase II GBM studies is presented in the table below:
|
DOSING REGIMEN & STUDY |
SINGLE DOSE |
Acute Regimen (single cycle) |
Comparative Cumulative Dose (@ 35 days) |
Dose Density (dose per week) |
Status |
|
|
NCI GBM historical regimen (Eagan etal) daily x 5 q 5wks (cycle = 35 days) |
25 mg/m2 |
x5 days = |
125 mg/m2 |
125 mg/m2 |
25mg/m2/wk |
Historical Studies: Myelosuppression observed |
|
DelMar VAL-083 regimen daily x 3 q 3wks (cycle = 21 days) |
30 mg/m2 |
x3 days = |
90 mg/m2 |
180 mg/m2 |
30mg/m2/wk |
No DLT |
|
40 mg/m2 |
120 mg/m2 |
240 mg/m2 |
40mg/m2/wk |
No DLT |
||
|
50 mg/m2 |
150 mg/m2 |
300 mg/m2 |
50mg/m2/wk |
ongoing |
||
|
60 mg/m2 |
180 mg/m2 |
360 mg/m2 |
60mg/m2/wk |
planned |
||
"We have now achieved significantly higher doses in comparison to the NCI regimen without encountering dose limiting toxicity," confirmed Mr. Bacha.
Safety Data Summary
DelMar reported that NCI-CTCAE Grade 1 lymphopenia (LLN to >3,000/mL) and thrombocytopenia (platelet counts LLN to >75,000/mL) at doses above 20mg/m2/day x 3 days had been observed in GBM treated to date in the current study; however, no serious adverse events related to study drug or dose limiting toxicity (DLT) has been encountered at doses up to 40mg/m2/day x 3 days.
A summary of hematologic-related safety data observed to date in the DelMar clinical trial is presented in the table below:
|
Cohort |
Dose & Hematologic Toxicity |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
Total |
|
1 - 4 |
up to 10 mg/m2 |
||||||
|
LYMPHOPENIA |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
THROMBOCYTOPENIA |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
5 |
20 mg/m2 |
||||||
|
LYMPHOPENIA |
1 (25%) |
0 |
0 |
0 |
0 |
1 (25%) |
|
|
THROMBOCYTOPENIA |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
6 |
30 mg/m2 |
||||||
|
LYMPHOPENIA |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
THROMBOCYTOPENIA |
1 (33%) |
0 |
0 |
0 |
0 |
1 (25%) |
|
|
7 |
40 mg/m2 |
||||||
|
LYMPHOPENIA |
1 (33%) |
0 |
0 |
0 |
0 |
1 (25%) |
|
|
THROMBOCYTOPENIA |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
8 |
50 mg/m2 |
Ongoing |
|||||
|
9 |
60 mg/m2 |
TBD if no DLT @ 50mg/m2 |
|||||
Mr. Bacha stated, "While low-grade thrombocytopenia and lymphopenia may signal that we are approaching the maximum tolerated dose, we believe that maximizing drug exposure and concentration at the tumor through exploration of higher doses will enhance patient outcomes."
"We are pleased that our modernized regimen is allowing us to achieve the goal to 'hit the tumor harder more often.' Based on the results to date, we filed a protocol amendment with the FDA and continued dose escalation under our protocol to 50mg/m2."
In accordance with the protocol, maximum tolerated dose (MTD) will be established by dose limiting toxicity (DLT), defined as:
- Hematologic DLT
- Grade 4 thrombocytopenia (platelets <25,000/mL), or Grade 3 thrombocytopenia (platelets <50,000 -25,000/mL with hemorrhage);
- Absolute neutrophil count (ANC) nadir < 500/mL or platelet count <50,000/mL, either lasting for more than five days;
- ANC < 500/mL with fever (febrile neutropenia); or
- Treatment delays of greater than 3 weeks for hematologic toxicity
- Non-hematologic DLT
- Any grade 3 or 4 non-hematologic toxicity due to treatment with the exception of alopecia, nausea, and vomiting;
- Grade 3 or 4 nausea or vomiting while receiving an optimal antiemetic regimen for prophylaxis and management;
- Treatment delays of greater than 3 weeks for toxicity
Pharmacokinetic Summary:
DelMar also reported updated pharmacokinetic analyses for cohorts 1-7, which demonstrate dose-dependent linear systemic exposure with a short plasma 1-2 h terminal half-life; Cmax at the highest dose tested (cohort 7, 40 mg/m2) ranged from 1130 to 739 ng/mL (7.7 to 5.1 uM). These data are correlated with the PK profile published in the scientific literature (Eagan et al 1982) as shown in the following figure:
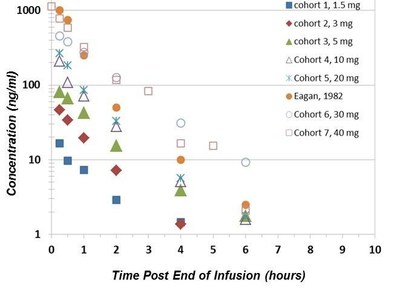
"One of the many benefits of the depth NCI-sponsored clinical trial data available to us is the opportunity to correlate our modern data with historical pharmacokinetics," said Mr. Bacha. "By extrapolating central nervous system (CNS) drug exposure from information in the published literature, we can calculate the expected CNS concentrations based on observed plasma concentrations of VAL-083 in our clinical trial."
DelMar reported that plasma drug concentrations predict that levels of VAL-083 in brain tissue exceed levels that have been shown to be effective against GBM cell lines in vitro.
"In other words, while we have not reached the maximum tolerated dose, the drug levels reaching the tumor already are predicted to exceed those shown to be effective in laboratory studies," added Mr. Bacha.
The following table illustrates the estimated concentration in of VAL-083 Human Brain tissue in the 40mg/m2 dose cohort compared to the concentration required for activity against GBM cell lines (IC50) in laboratory studies.
|
Dose: Each Day of Cycle in Current Trial |
Plasma Cmax (ug/mL)1 |
Estimated Maximum Tumor Concentration in Brain 2,3 |
IC50 in GBM Cell Lines |
|
|
(ug/g tissue) |
uM* |
uM |
||
|
40mg/m2 Day-1 |
0.781 |
0.344 |
2.36 |
2.5-5.0 |
|
40mg/m2 Day-2 |
0.781 |
0.503 |
3.45 |
2.5-5.0 |
|
40mg/m2 Day-3 |
0.781 |
0.563 |
3.86 |
2.5-5.0 |
Anti-tumor Activity and Next Steps
The goal of DelMar's Phase I/II trial is to establish the maximum tolerated dose (MTD) of VAL-083 in a modernized dosing regimen for advancement into registration directed trials as a potential new therapy for the treatment of refractory GBM.
Patients enrolled have recurrent GBM that has failed to respond to prior therapy. Cycle 1 toxicity is measured for determination of MTD. Tumor volume is measured via Response Assessment in Neuro-Oncology (RANO) criteria prior to every other 21-day treatment cycle and patients exhibiting stable disease or tumor regression allowed to remain on study drug. Three patients exhibiting a response (stable disease or partial response) reported improved clinical signs with a maximum response of 28 cycles (84 weeks) prior to discontinuing due to adverse events unrelated to the study.
To date, no drug-related serious adverse events have been detected and MTD has not been reached at doses up to 40mg/m2. DelMar is currently studying a dose of 50mg/m2 in this clinical trial. The current clinical protocol requires acquisition of safety data for 35 days following initial treatment with VAL-083 in three patients to meet the primary endpoint for determination of MTD. While patients have been identified for the 50 mg/m2 cohort, none have yet completed the required 35 day follow-up period. Normal instances in clinical development such as patient ineligibility at screening, failure to obtain patient consent or patient death prior to 35 days following dosing may require identification and recruitment of replacement patients. Once 35-day safety data from three patients in the 50mg/m2 cohort is obtained, review of data will either confirm MTD or allow advancement to higher doses.
"We will work with our clinical advisors to obtain and analyze these data in the timeliest manner possible," said Mr. Bacha. "Based on our current enrollment and timelines, we believe we remain on track to advance to registration directed trials with VAL-083 in the first half of 2015."
About VAL-083
VAL-083 is a first-in-class, small-molecule chemotherapeutic with a unique mechanism of action. In more than 40 Phase 1 and 2 clinical studies sponsored by the National Cancer Institute, VAL-083 has shown safety and efficacy in treating a number of cancers including lung, brain, cervical, ovarian tumors and leukemia. VAL-083 is approved in China for the treatment of chronic myelogenous leukemia and lung cancer and has received orphan drug designation in Europe and the U.S. for the treatment of gliomas. As a potential treatment for glioblastoma, VAL-083's mechanism of action is unaffected by the expression of MGMT, a DNA repair enzyme that causes chemotherapy resistance to front-line treatment with Temodar® (temozolomide). DelMar is currently studying VAL-083 in a Phase 1/2 clinical trial for patients with refractory glioblastoma multiforme.
About Glioblastoma Multiforme (GBM)
Glioblastoma multiforme (GBM) is the most common and most malignant form of brain cancer. Approximately 15,000 people are diagnosed with GBM each year in the U.S., with similar incidence in Europe. Standard of care is surgery, followed by either radiation therapy, or radiation therapy combined with temozolomide. Approximately 60 percent of GBM patients treated with temozolomide experience tumor progression within one year. More than half of glioblastoma patients will fail the currently approved therapies and face a very poor prognosis.
About the Society for Neuro-Oncology (SNO) annual meeting
The Society for Neuro-Oncology is a multidisciplinary organization dedicated to promoting advances in neuro-oncology through research and education. It is the premier North American organization for clinicians, basic scientists, nurses and other health care professionals whose focus is central nervous system tumors in children and adults. The 19th Annual Scientific Meeting and Education Day was held November 13-16, 2014, at the Loews Hotel South Beach in Miami.
About DelMar Pharmaceuticals, Inc.
DelMar Pharmaceuticals, Inc. was founded to develop and commercialize proven cancer therapies in new orphan drug indications where patients are failing or have become intolerable to modern targeted or biologic treatments. The Company's lead drug in development, VAL-083, is currently undergoing clinical trials in the U.S. as a potential treatment for refractory glioblastoma multiforme. VAL-083 has been extensively studied by U.S. National Cancer Institute, and is currently approved for the treatment of chronic myelogenous leukemia (CML) and lung cancer in China. Published pre-clinical and clinical data suggest that VAL-083 may be active against a range of tumor types via a novel mechanism of action that could provide improved treatment options for patients.
For more information, please visit www.delmarpharma.com or follow us on Twitter @delmarpharma or Facebook.com/delmarpharma.
Safe Harbor Statement
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. Any forward-looking statements contained herein are based on current expectations, but are subject to a number of risks and uncertainties. The factors that could cause actual future results to differ materially from current expectations include, but are not limited to, risks and uncertainties relating to the Company's ability to develop, market and sell products based on its technology; the expected benefits and efficacy of the Company's products and technology; the availability of substantial additional funding for the Company to continue its operations and to conduct research and development, clinical studies and future product commercialization; and, the Company's business, research, product development, regulatory approval, marketing and distribution plans and strategies. These and other factors are identified and described in more detail in our filings with the SEC, including, our current reports on Form 8-K. We do not undertake to update these forward-looking statements made by us.
Photo - https://photos.prnewswire.com/prnh/20141117/158912-INFO
SOURCE DelMar Pharmaceuticals, Inc.
Released November 17, 2014