
Overcoming Resistance to Cancer Immunotherapy Presentation | March 2025 tuhurabio.com Filed by TuHURA Biosciences, Inc. Pursuant to Rule 425 under the Securities Act of 1933 and deemed filed pursuant to Rule 14a-12 under the Securities Act of 1934 Filer: TuHURA Biosciences, Inc. Commission File No.: 001-37823 Subject Company: Kineta, Inc. Commission File No.: 001-37695 Date: March 18, 2025

Forward-Looking Statements This presentation includes “forward-looking statements” under the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, and TuHURA’s actual results may differ from its expectations, estimates and projections expressed in its forward-looking statements, and consequently you should not rely on these forward-looking statements as predictions of future events. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue,” and similar expressions are intended to identify such forward-looking statements. These forward-looking statements include, without limitation, statements about TuHURA’s IFx-Hu2.0 product candidate, its IFx-Hu3.0 preclinical program, its tumor microenvironment modulators development program, its potential acquisition by merger of Kineta Inc. and the statements about Kineta’s VISTA-101 development program, and any developments or results in connection therewith and the anticipated regulatory pathway and timing of those development programs, studies and trials. These forward-looking statements involve significant risks and uncertainties that could cause the actual results to differ materially from the expected results, including the risks set forth in the “Risk Factors” sections of TuHURA’s most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q , and other documents filed by TuHURA from time wo time with the Securities and Exchange Commission. TuHURA does not undertake or accept any obligation or undertaking to update or revise any forward-looking statements to reflect any change in its expectations or any change in events, conditions or circumstances on which any such statement is based. Disclaimer Additional Information about the Proposed Mergers and Where to Find It This communication may be deemed to be solicitation material with respect to the proposed transactions between TuHURA and Kineta, Inc. (“Kineta”). In connection with the proposed transactions, TuHURA intends to file relevant materials with the SEC. TuHURA will mail the joint proxy statement/prospectus to the TuHURA stockholders. Investors and securityholders of TuHURA and Kineta are urged to read these materials when they become available because they will contain important information about TuHURA, Kineta and the proposed transactions. This communication is not a substitute for the definitive proxy statement/prospectus, when it becomes available, or any other documents that TuHURA may file with the SEC or send to securityholders in connection with the proposed transactions. Investors and stockholders will be able to obtain free copies of the documents filed or that will be filed with the SEC by TuHURA, when they become available, through the website maintained by the SEC at www.sec.gov. The documents filed by TuHURA with the SEC may also be obtained free of charge at TuHURA’s website at www.tuhurabio.com or upon written request to: TuHURA, 10500 University Drive, Suite 110, Tampa, Florida 33612. No Offer or Solicitation This investor presentation is not a proxy statement or solicitation of a proxy, consent or authorization with respect to any securities or in respect of the potential transaction and is not intended to and does not constitute an offer to sell or the solicitation of an offer to buy the securities of TuHURA or Kineta, nor shall there be any sale of any such securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act. Participants in the Solicitation TuHURA and Kineta and their respective directors and officers and other members of management may, under SEC rules, be deemed to be participants in the solicitation of proxies from stockholders in connection with the potential transaction and other matters that may be set forth in the proxy statement/prospectus. Information about TuHURA’s directors and executive officers is set forth in TuHURA’s filings with the SEC, including TuHURA’s Current Report on Form 8-K filed with the SEC on October 21, 2024. Additional information regarding the direct and indirect interests, by security holdings or otherwise, of those persons and other persons who may be deemed participants in the solicitation of proxies in the potential transaction may be obtained by reading the proxy statement/prospectus when it becomes available. You may obtain free copies of these documents as described above under “Additional Information about the Proposed Mergers and Where to Find It ”.

Investment Summary * Trial currently subject to partial clinical hold relating to completion of certain CMC requirements for initiation of Phase 3 registration trial MCC = Merkel cell carcinoma Business Highlights Tumor Microenvironment Modulators Innate Immune Agonists Single Phase 3 registration directed Accelerated Approval trial for IFx-2.0* Demonstrated durable CRs and PRs in patients progressing on CPI in Phase 2 trial FDA Project Front Runner initiative encouraged trial in first line setting SPA Agreement with FDA on novel trial design could remove requirement for post approval confirmatory trial, potentially converting accelerated approval to full approval Top-line data anticipated 2H 2026 Building a de-risked late-stage product pipeline Definitive agreement to acquire by merger Kineta’s VISTA inhibiting mAb in clinical partnership with Merck If completed, Kineta transaction adds Phase 2 stage candidate to development pipeline in NMP1 mutated AML First-in-class, non-tumor targeting, bi-specific immune modulating ADCs/APCs Clinical, corporate and regulatory milestones with 4 key data readouts expected over the next 24 months
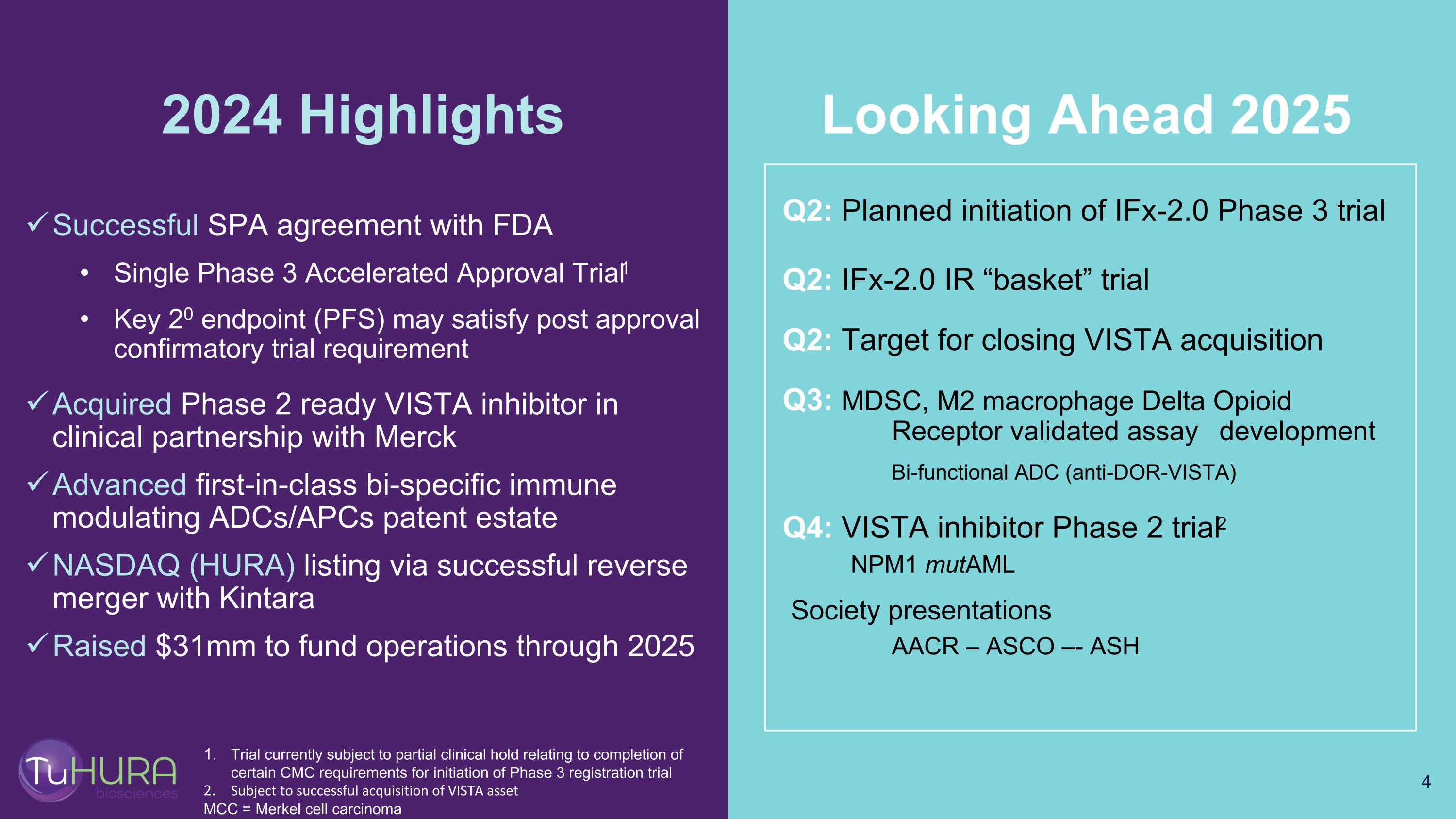
Successful SPA agreement with FDA Single Phase 3 Accelerated Approval Trial1 Key 20 endpoint (PFS) may satisfy post approval confirmatory trial requirement Acquired Phase 2 ready VISTA inhibitor in clinical partnership with Merck Advanced first-in-class bi-specific immune modulating ADCs/APCs patent estate NASDAQ (HURA) listing via successful reverse merger with Kintara Raised $31mm to fund operations through 2025 2024 Highlights Q2: Planned initiation of IFx-2.0 Phase 3 trial Q2: IFx-2.0 IR “basket” trial Q2: Target for closing VISTA acquisition Q3: MDSC, M2 macrophage Delta Opioid Receptor validated assay development Bi-functional ADC (anti-DOR-VISTA) Q4: VISTA inhibitor Phase 2 trial2 NPM1 mutAML Society presentations AACR – ASCO –- ASH Looking Ahead 2025 Trial currently subject to partial clinical hold relating to completion of certain CMC requirements for initiation of Phase 3 registration trial Subject to successful acquisition of VISTA asset MCC = Merkel cell carcinoma
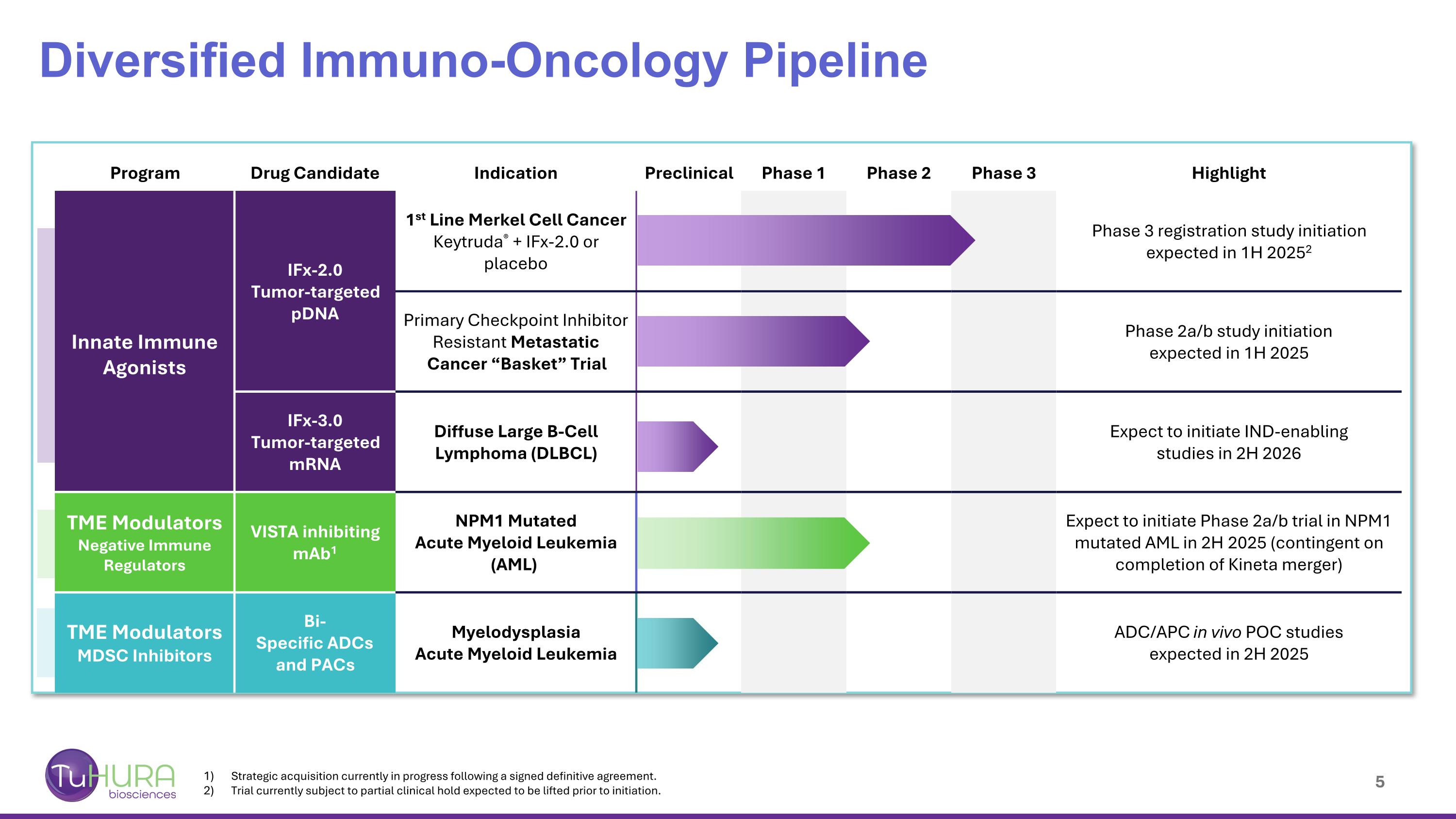
Diversified Immuno-Oncology Pipeline Program Drug Candidate Indication Preclinical Phase 1 Phase 2 Phase 3 Highlight Innate Immune Agonists IFx-2.0 Tumor-targeted pDNA 1st Line Merkel Cell Cancer Keytruda® + IFx-2.0 or placebo Phase 3 registration study initiation expected in 1H 20252 IFx-Hu2.0 Primary Checkpoint Inhibitor Resistant Metastatic Cancer “Basket” Trial Phase 2a/b study initiation expected in 1H 2025 IFx-3.0 Tumor-targeted mRNA Diffuse Large B-Cell Lymphoma (DLBCL) Expect to initiate IND-enabling studies in 2H 2026 TME Modulators Negative Immune Regulators VISTA inhibiting mAb1 NPM1 Mutated Acute Myeloid Leukemia (AML) Expect to initiate Phase 2a/b trial in NPM1 mutated AML in 2H 2025 (contingent on completion of Kineta merger) TME Modulators MDSC Inhibitors Bi- Specific ADCs and PACs Myelodysplasia Acute Myeloid Leukemia ADC/APC in vivo POC studies expected in 2H 2025 Strategic acquisition currently in progress following a signed definitive agreement. Trial currently subject to partial clinical hold expected to be lifted prior to initiation.

IFx Technology Innate Immune Agonists Designed to Overcome Primary Resistance to Checkpoint Inhibitors
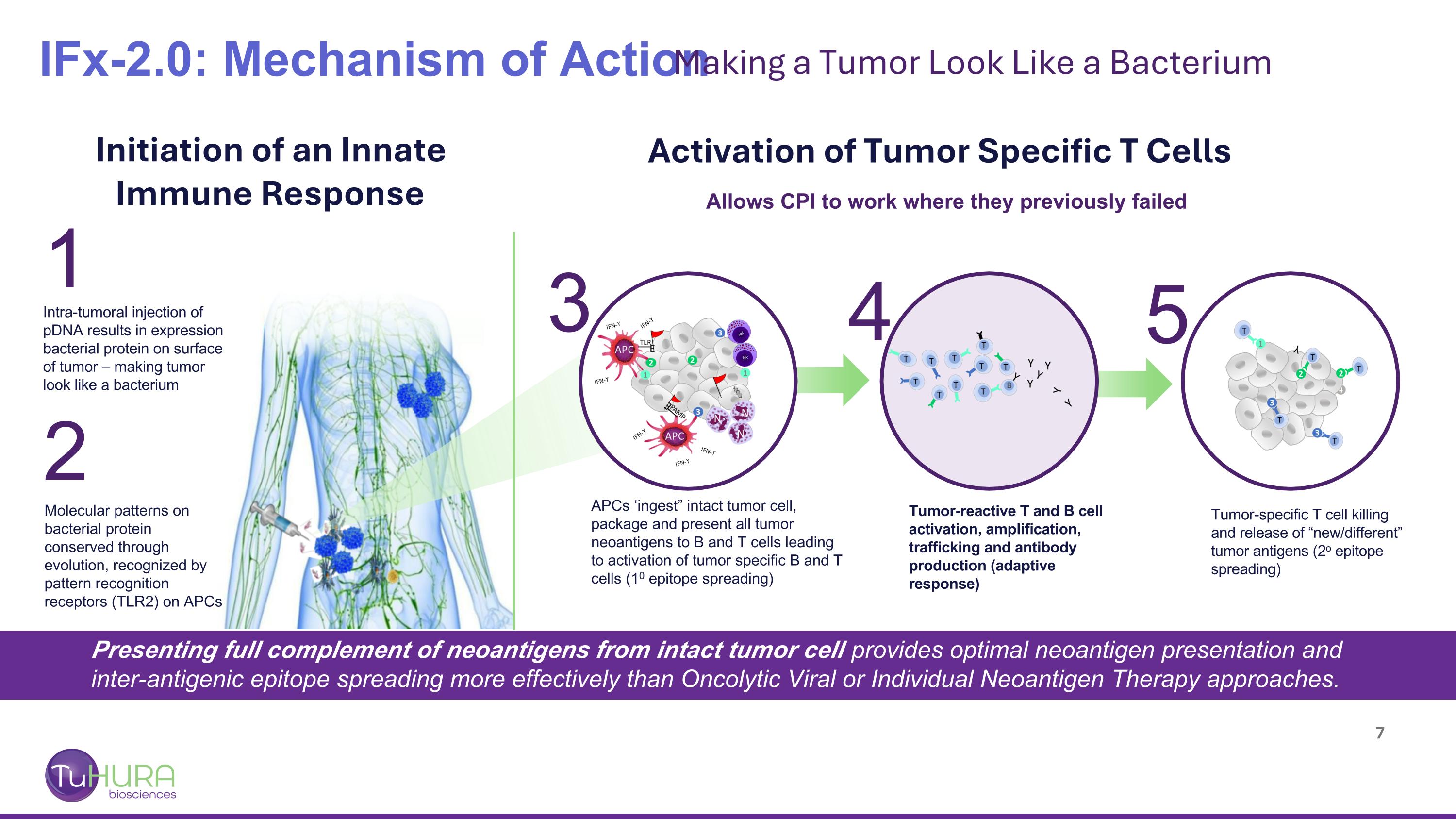
3 IFx-2.0: Mechanism of Action Making a Tumor Look Like a Bacterium Initiation of an Innate Immune Response Activation of Tumor Specific T Cells Intra-tumoral injection of pDNA results in expression bacterial protein on surface of tumor – making tumor look like a bacterium Molecular patterns on bacterial protein conserved through evolution, recognized by pattern recognition receptors (TLR2) on APCs Tumor-reactive T and B cell activation, amplification, trafficking and antibody production (adaptive response) Tumor-specific T cell killing and release of “new/different” tumor antigens (2o epitope spreading) APCs ‘ingest” intact tumor cell, package and present all tumor neoantigens to B and T cells leading to activation of tumor specific B and T cells (10 epitope spreading) Allows CPI to work where they previously failed Presenting full complement of neoantigens from intact tumor cell provides optimal neoantigen presentation and inter-antigenic epitope spreading more effectively than Oncolytic Viral or Individual Neoantigen Therapy approaches. 1 2 4 5
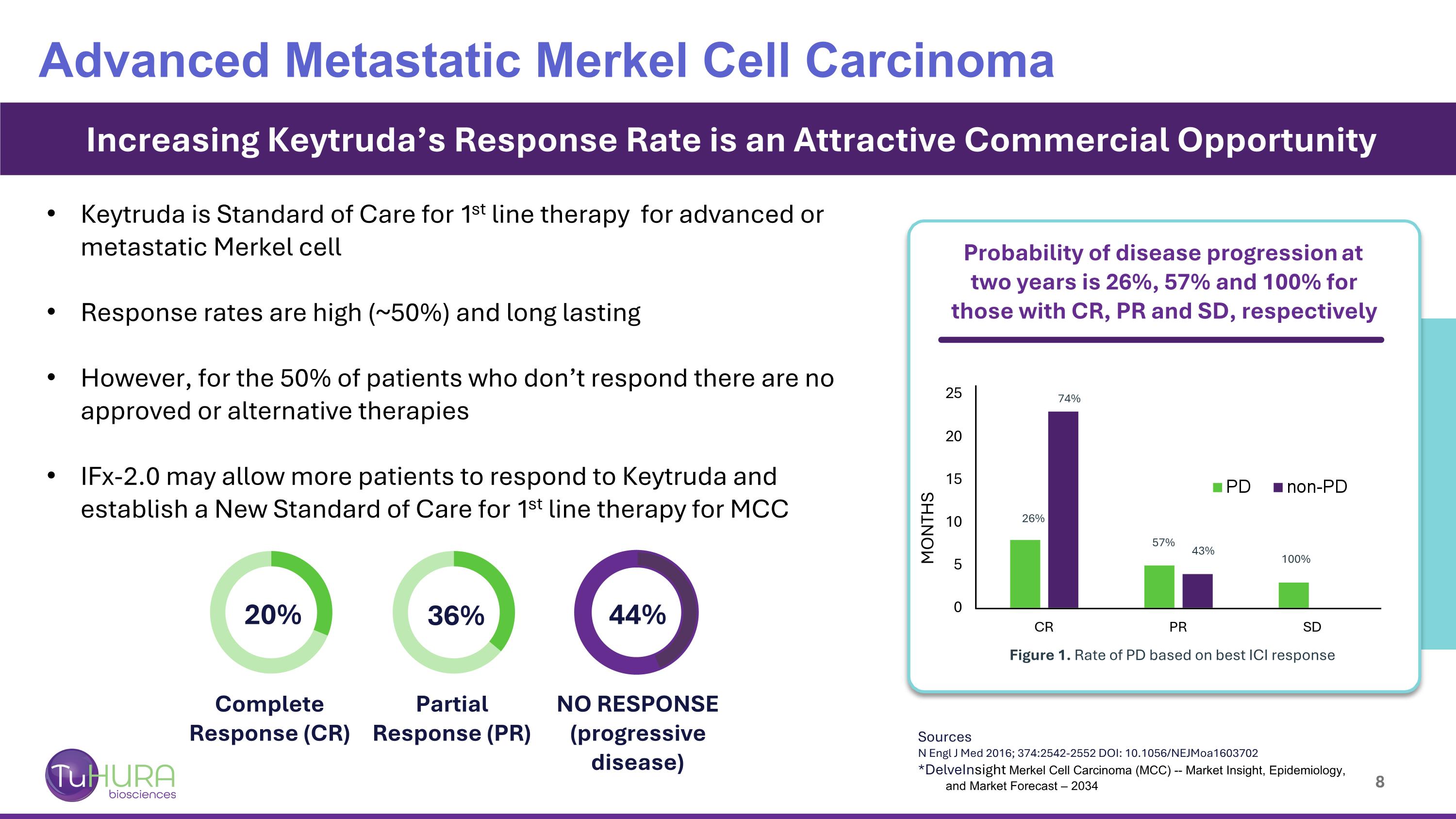
Sources N Engl J Med 2016; 374:2542-2552 DOI: 10.1056/NEJMoa1603702 *DelveInsight Merkel Cell Carcinoma (MCC) -- Market Insight, Epidemiology, and Market Forecast – 2034 Increasing Keytruda’s Response Rate is an Attractive Commercial Opportunity Probability of disease progression at two years is 26%, 57% and 100% for those with CR, PR and SD, respectively 26% 74% 57% 43% 100% Figure 1. Rate of PD based on best ICI response Complete Response (CR) Partial Response (PR) NO RESPONSE (progressive disease) 20% 36% 44% Advanced Metastatic Merkel Cell Carcinoma MONTHS Keytruda is Standard of Care for 1st line therapy for advanced or metastatic Merkel cell Response rates are high (~50%) and long lasting However, for the 50% of patients who don’t respond there are no approved or alternative therapies IFx-2.0 may allow more patients to respond to Keytruda and establish a New Standard of Care for 1st line therapy for MCC
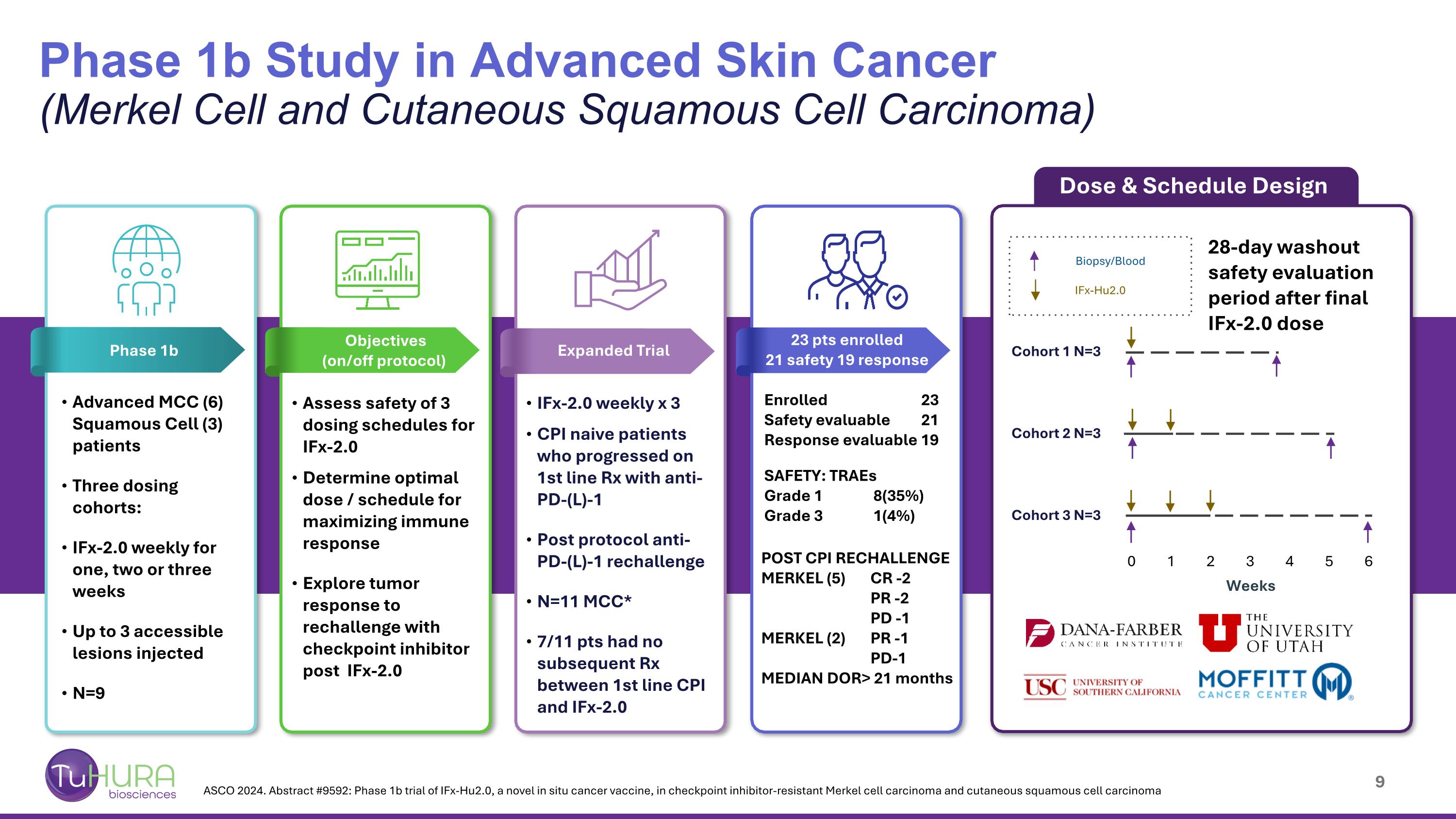
Phase 1b Study in Advanced Skin Cancer (Merkel Cell and Cutaneous Squamous Cell Carcinoma) Advanced MCC (6) Squamous Cell (3) patients Three dosing cohorts: IFx-2.0 weekly for one, two or three weeks Up to 3 accessible lesions injected N=9 IFx-Hu2.0 Biopsy/Blood Cohort 1 N=3 Cohort 2 N=3 Cohort 3 N=3 Weeks 0 1 2 3 4 5 6 Dose & Schedule Design IFx-2.0 weekly x 3 CPI naive patients who progressed on 1st line Rx with anti-PD-(L)-1 Post protocol anti-PD-(L)-1 rechallenge N=11 MCC* 7/11 pts had no subsequent Rx between 1st line CPI and IFx-2.0 Assess safety of 3 dosing schedules for IFx-2.0 Determine optimal dose / schedule for maximizing immune response Explore tumor response to rechallenge with checkpoint inhibitor post IFx-2.0 28-day washout safety evaluation period after final IFx-2.0 dose Enrolled 23 Safety evaluable 21 Response evaluable 19 SAFETY: TRAEs Grade 1 8(35%) Grade 3 1(4%) POST CPI RECHALLENGE MERKEL (5) CR -2 PR -2 PD -1 MERKEL (2) PR -1 PD-1 MEDIAN DOR> 21 months Objectives (on/off protocol) Phase 1b Expanded Trial 23 pts enrolled 21 safety 19 response ASCO 2024. Abstract #9592: Phase 1b trial of IFx-Hu2.0, a novel in situ cancer vaccine, in checkpoint inhibitor-resistant Merkel cell carcinoma and cutaneous squamous cell carcinoma
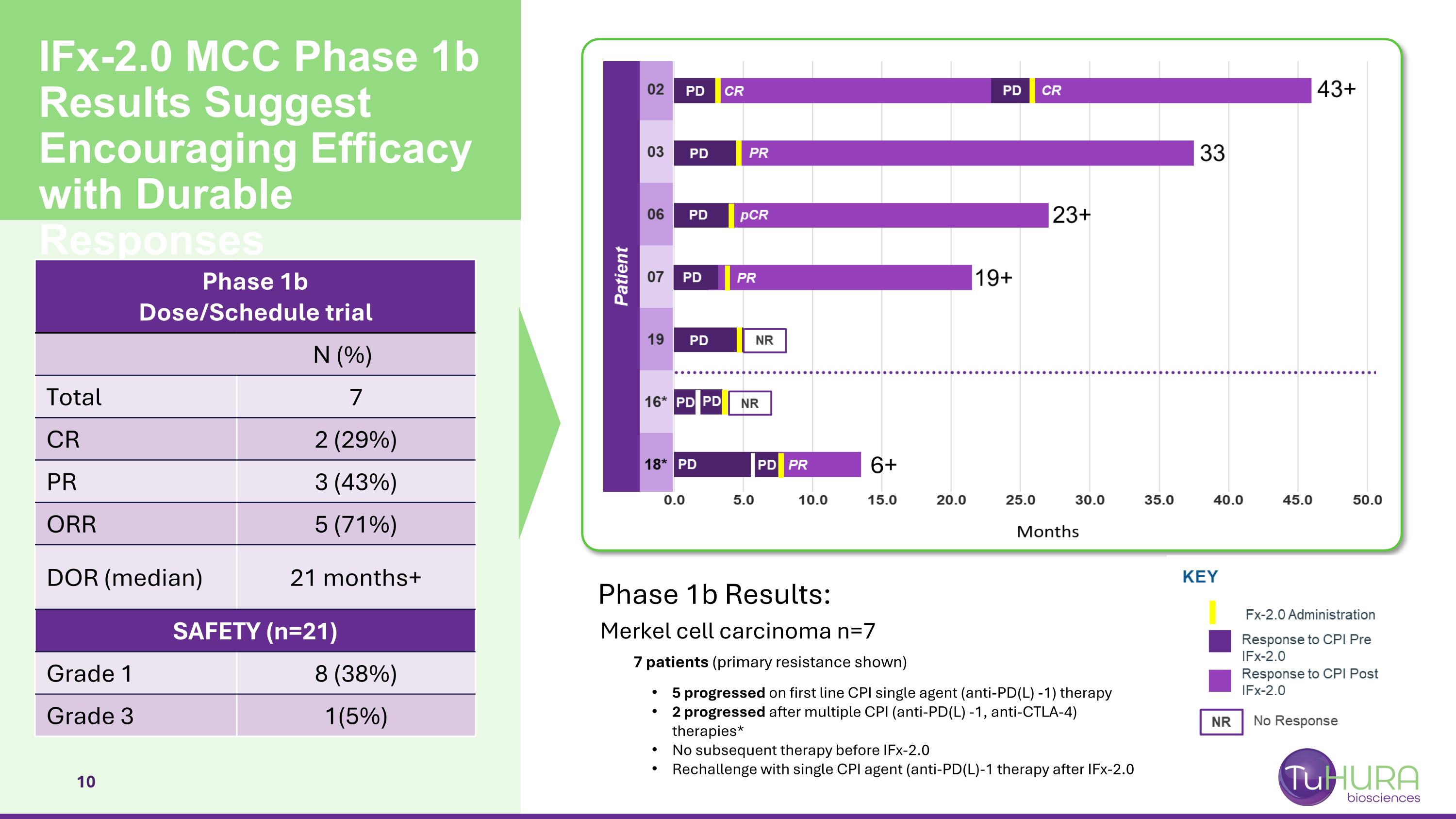
IFx-2.0 MCC Phase 1b Results Suggest Encouraging Efficacy with Durable Responses Phase 1b Dose/Schedule trial N (%) Total 7 CR 2 (29%) PR 3 (43%) ORR 5 (71%) DOR (median) 21 months+ SAFETY (n=21) Grade 1 8 (38%) Grade 3 1(5%) Phase 1b Results: Merkel cell carcinoma n=7 7 patients (primary resistance shown) 5 progressed on first line CPI single agent (anti-PD(L) -1) therapy 2 progressed after multiple CPI (anti-PD(L) -1, anti-CTLA-4) therapies* No subsequent therapy before IFx-2.0 Rechallenge with single CPI agent (anti-PD(L)-1 therapy after IFx-2.0 6+
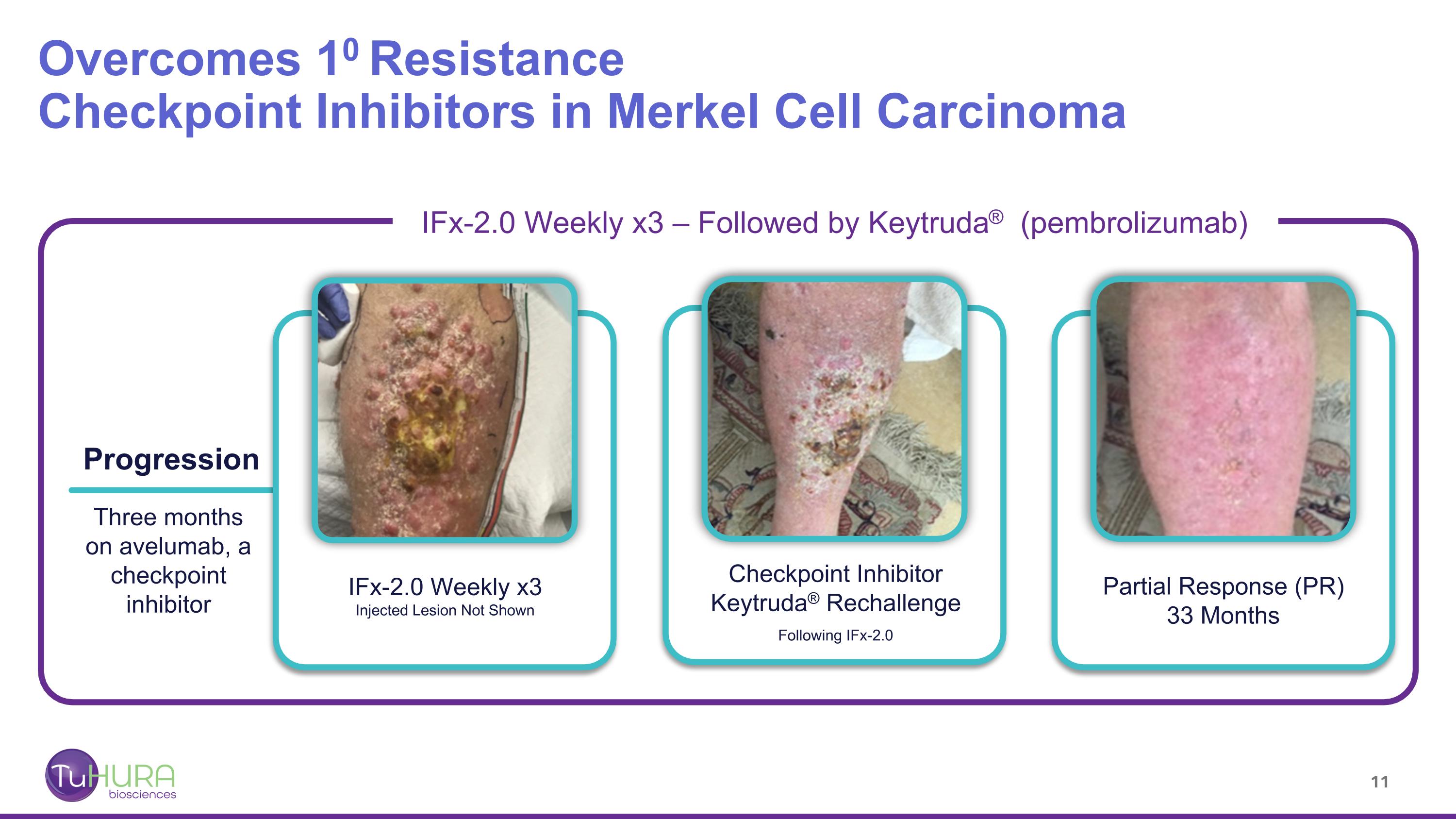
Overcomes 10 Resistance Checkpoint Inhibitors in Merkel Cell Carcinoma Progression Three months on avelumab, a checkpoint inhibitor IFx-2.0 Weekly x3 Injected Lesion Not Shown Checkpoint Inhibitor Keytruda® Rechallenge Following IFx-2.0 Partial Response (PR) 33 Months IFx-2.0 Weekly x3 – Followed by Keytruda® (pembrolizumab)
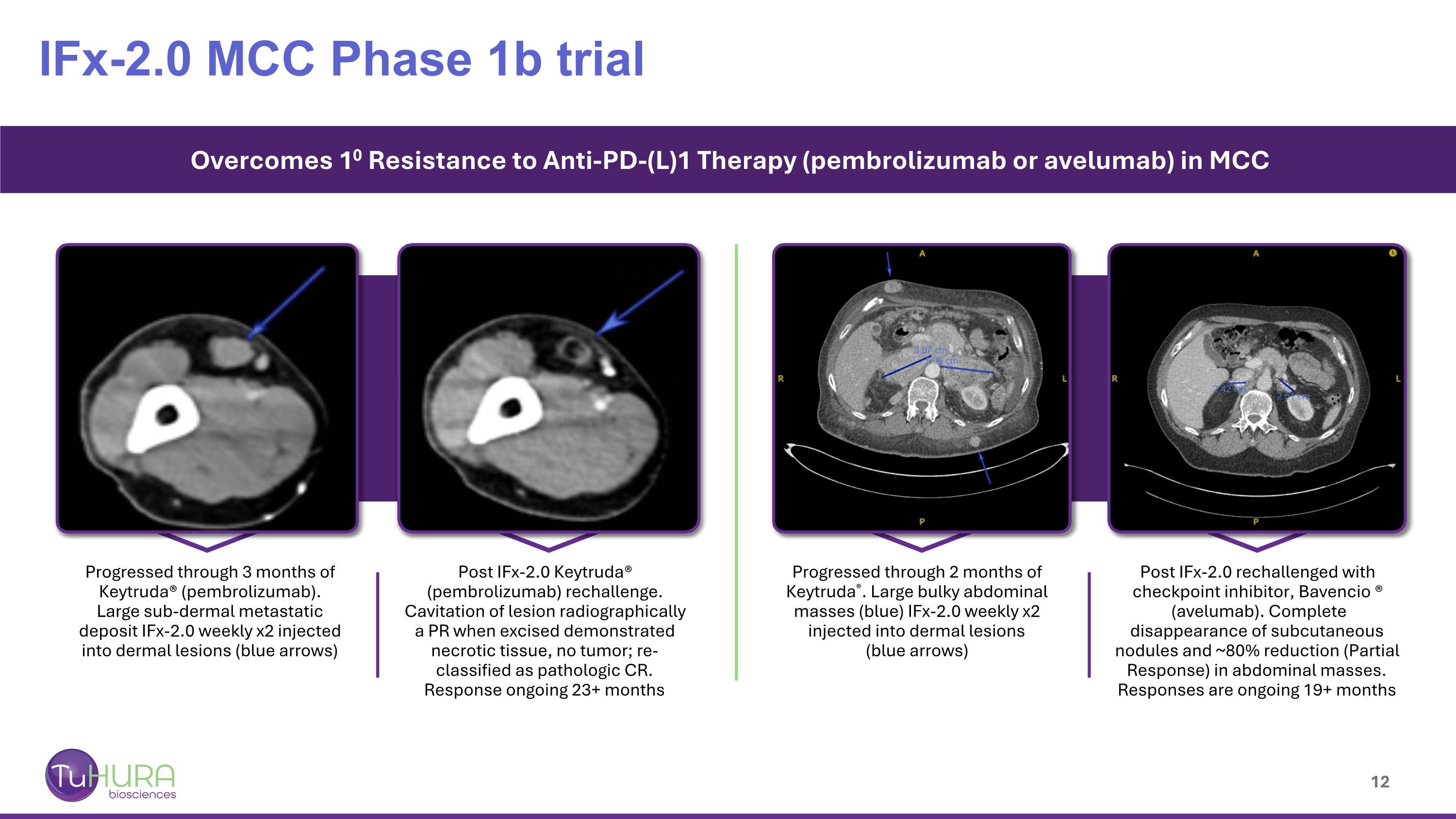
IFx-2.0 MCC Phase 1b trial Overcomes 10 Resistance to Anti-PD-(L)1 Therapy (pembrolizumab or avelumab) in MCC Progressed through 3 months of Keytruda® (pembrolizumab). Large sub-dermal metastatic deposit IFx-2.0 weekly x2 injected into dermal lesions (blue arrows) Post IFx-2.0 Keytruda® (pembrolizumab) rechallenge. Cavitation of lesion radiographically a PR when excised demonstrated necrotic tissue, no tumor; re-classified as pathologic CR. Response ongoing 23+ months Progressed through 2 months of Keytruda®. Large bulky abdominal masses (blue) IFx-2.0 weekly x2 injected into dermal lesions (blue arrows) Post IFx-2.0 rechallenged with checkpoint inhibitor, Bavencio ® (avelumab). Complete disappearance of subcutaneous nodules and ~80% reduction (Partial Response) in abdominal masses. Responses are ongoing 19+ months
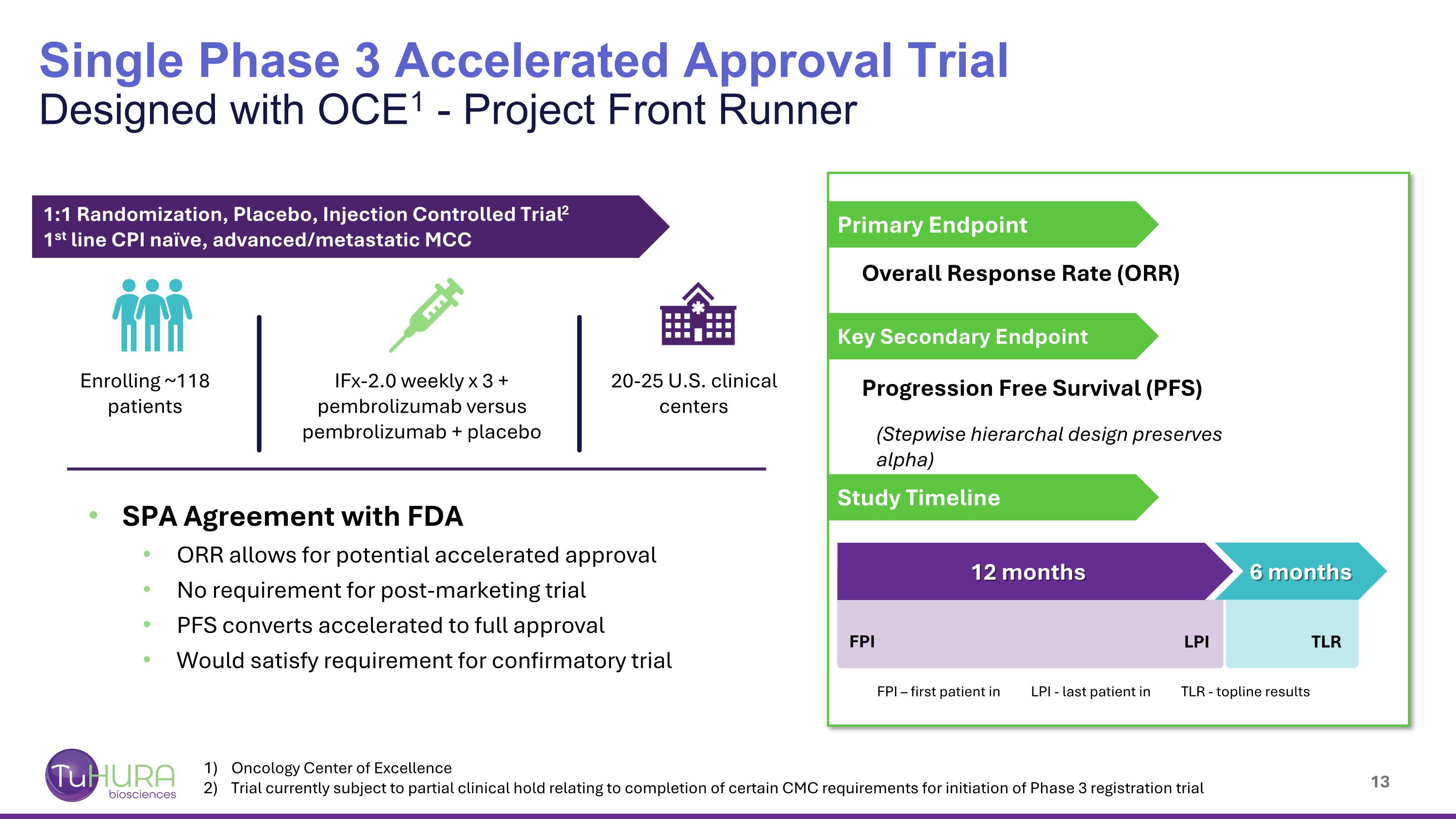
Oncology Center of Excellence Trial currently subject to partial clinical hold relating to completion of certain CMC requirements for initiation of Phase 3 registration trial Single Phase 3 Accelerated Approval Trial Designed with OCE1 - Project Front Runner FPI – first patient in LPI - last patient in TLR - topline results 1:1 Randomization, Placebo, Injection Controlled Trial2 1st line CPI naïve, advanced/metastatic MCC Enrolling ~118 patients IFx-2.0 weekly x 3 + pembrolizumab versus pembrolizumab + placebo 20-25 U.S. clinical centers SPA Agreement with FDA ORR allows for potential accelerated approval No requirement for post-marketing trial PFS converts accelerated to full approval Would satisfy requirement for confirmatory trial Overall Response Rate (ORR) Progression Free Survival (PFS) (Stepwise hierarchal design preserves alpha) Primary Endpoint Key Secondary Endpoint Study Timeline FPI LPI TLR 6 months 12 months
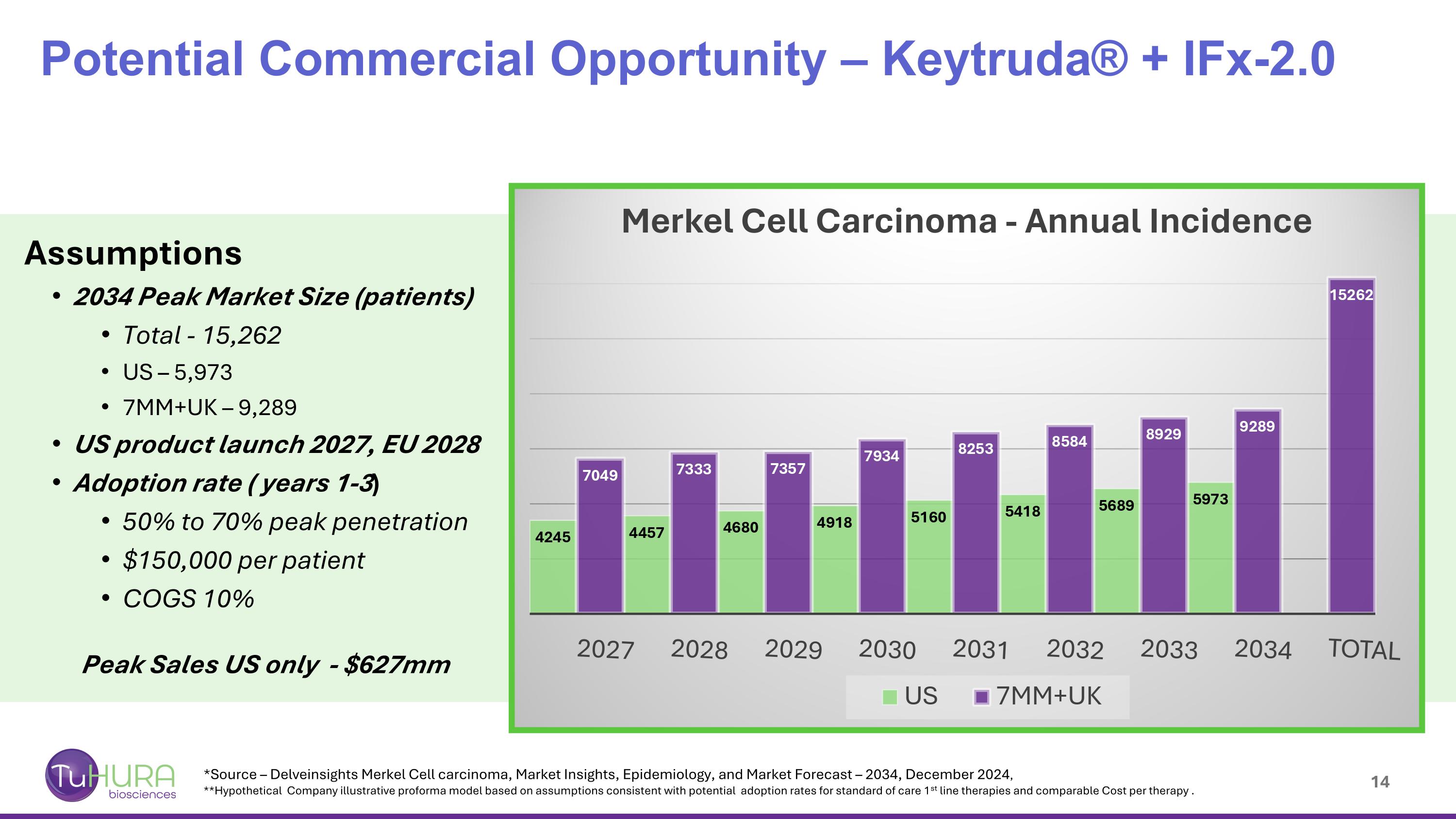
Potential Commercial Opportunity – Keytruda® + IFx-2.0 Assumptions 2034 Peak Market Size (patients) Total - 15,262 US – 5,973 7MM+UK – 9,289 US product launch 2027, EU 2028 Adoption rate ( years 1-3) 50% to 70% peak penetration $150,000 per patient COGS 10% Peak Sales US only - $627mm *Source – Delveinsights Merkel Cell carcinoma, Market Insights, Epidemiology, and Market Forecast – 2034, December 2024, **Hypothetical Company illustrative proforma model based on assumptions consistent with potential adoption rates for standard of care 1st line therapies and comparable Cost per therapy .
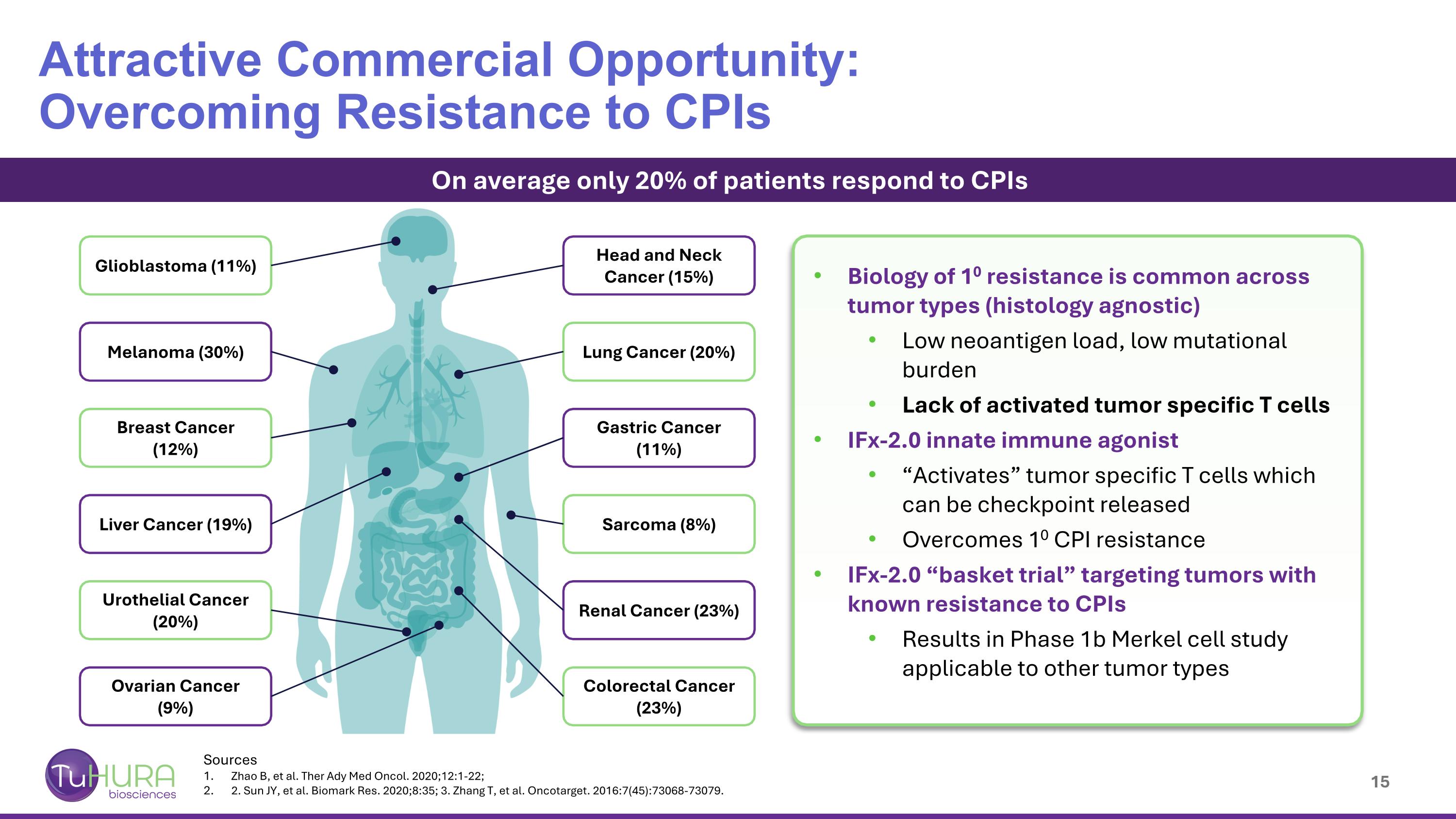
Sources Zhao B, et al. Ther Ady Med Oncol. 2020;12:1-22; 2. Sun JY, et al. Biomark Res. 2020;8:35; 3. Zhang T, et al. Oncotarget. 2016:7(45):73068-73079. Attractive Commercial Opportunity: Overcoming Resistance to CPIs On average only 20% of patients respond to CPIs Head and Neck Cancer (15%) Lung Cancer (20%) Gastric Cancer (11%) Sarcoma (8%) Renal Cancer (23%) Colorectal Cancer (23%) Ovarian Cancer (9%) Urothelial Cancer (20%) Liver Cancer (19%) Breast Cancer (12%) Melanoma (30%) Glioblastoma (11%) Biology of 10 resistance is common across tumor types (histology agnostic) Low neoantigen load, low mutational burden Lack of activated tumor specific T cells IFx-2.0 innate immune agonist “Activates” tumor specific T cells which can be checkpoint released Overcomes 10 CPI resistance IFx-2.0 “basket trial” targeting tumors with known resistance to CPIs Results in Phase 1b Merkel cell study applicable to other tumor types
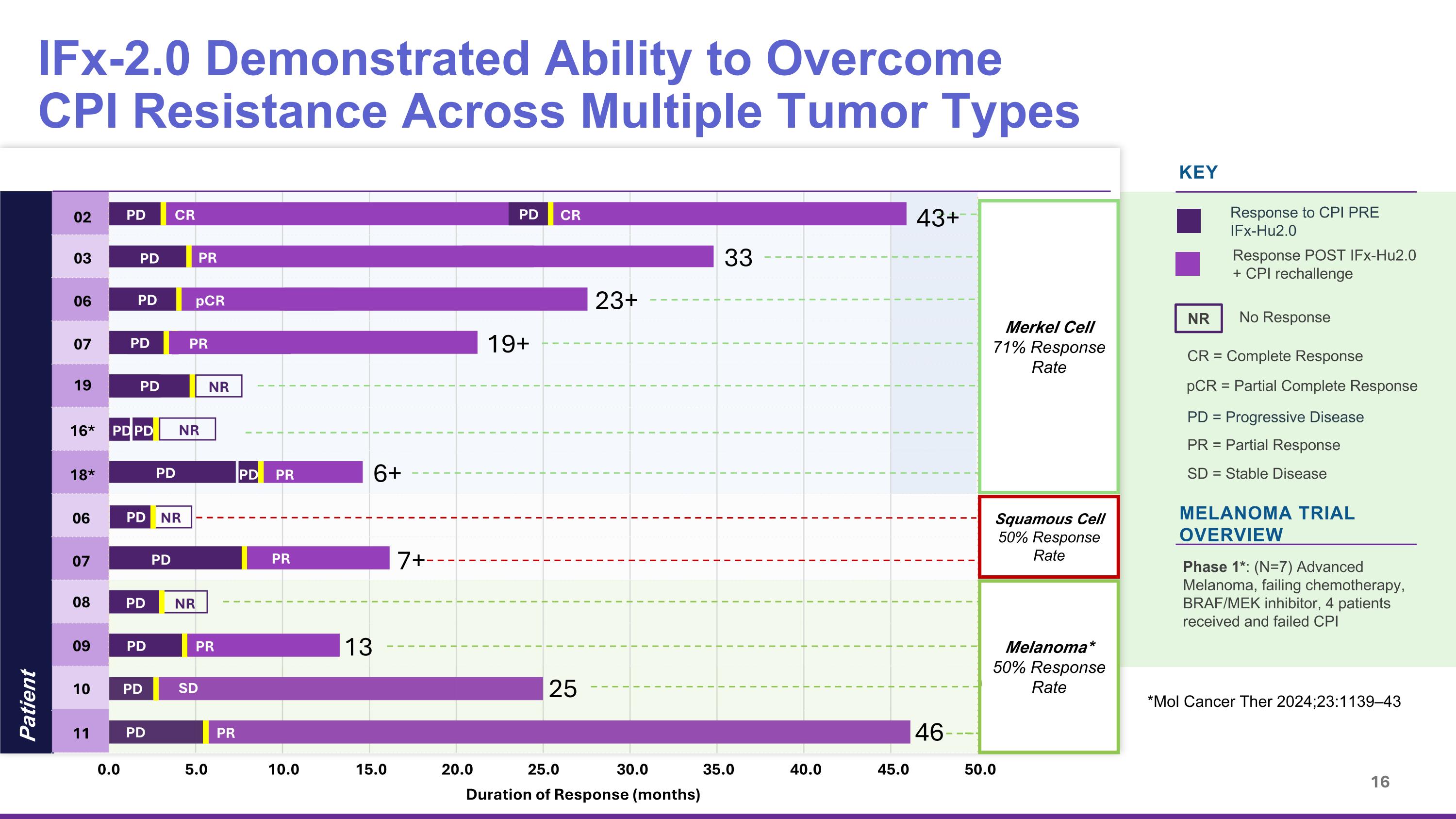
IFx-2.0 Demonstrated Ability to Overcome CPI Resistance Across Multiple Tumor Types Phase 1/1b Trials in Advanced Merkel Cell, Squamous Cell, Melanoma KEY Response POST IFx-Hu2.0 + CPI rechallenge Response to CPI PRE IFx-Hu2.0 No Response NR MELANOMA TRIAL OVERVIEW Phase 1*: (N=7) Advanced Melanoma, failing chemotherapy, BRAF/MEK inhibitor, 4 patients received and failed CPI SD = Stable Disease PR = Partial Response CR = Complete Response pCR = Partial Complete Response PD = Progressive Disease *Mol Cancer Ther 2024;23:1139–43 Patient Merkel Cell 71% Response Rate Melanoma* 50% Response Rate Squamous Cell 50% Response Rate PD CR PD CR PD PR PD pCR PD PR PD NR PD NR PD PD PD PR 02 03 06 07 19 16* 18* 43+ 33 23+ 19+ 0.0 5.0 10.0 15.0 45.0 40.0 20.0 25.0 30.0 35.0 50.0 13 25 46 6+ Duration of Response (months) PD PR PD SD PD PR NR 09 NR 11 10 PD PD PD PR 08 06 07 7+

Tumor Microenvironment Modulators Novel Targets for Intervention Negative Immune Regulators (VISTA) MDSCs and M2 macrophages (DOR inhibitors*) *DOR – Delta Opioid Receptor
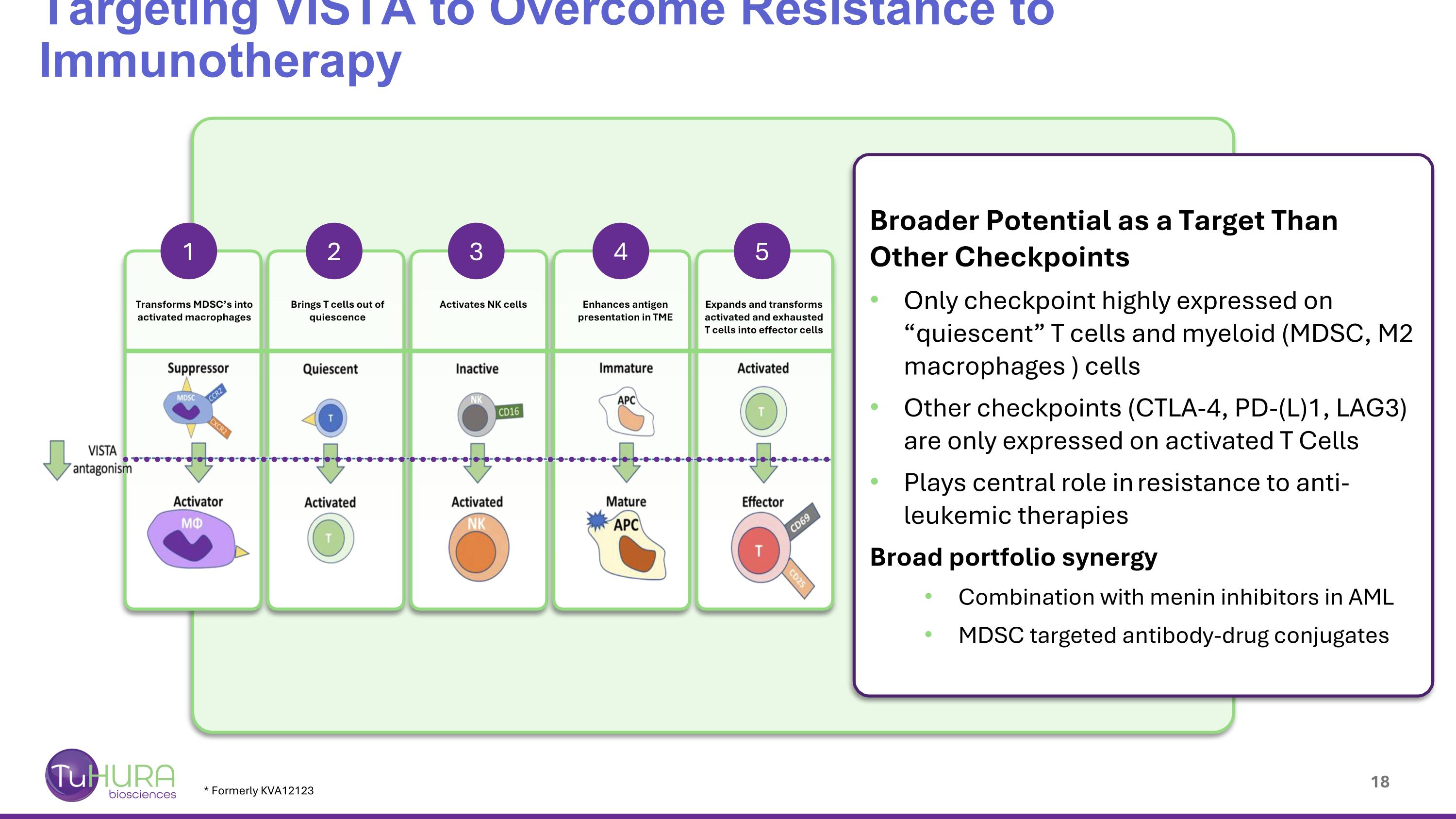
* Formerly KVA12123 Targeting VISTA to Overcome Resistance to Immunotherapy Broader Potential as a Target Than Other Checkpoints Only checkpoint highly expressed on “quiescent” T cells and myeloid (MDSC, M2 macrophages ) cells Other checkpoints (CTLA-4, PD-(L)1, LAG3) are only expressed on activated T Cells Plays central role in resistance to anti-leukemic therapies Broad portfolio synergy Combination with menin inhibitors in AML MDSC targeted antibody-drug conjugates 1 2 3 4 5 Transforms MDSC’s into activated macrophages Brings T cells out of quiescence Activates NK cells Enhances antigen presentation in TME Expands and transforms activated and exhausted T cells into effector cells
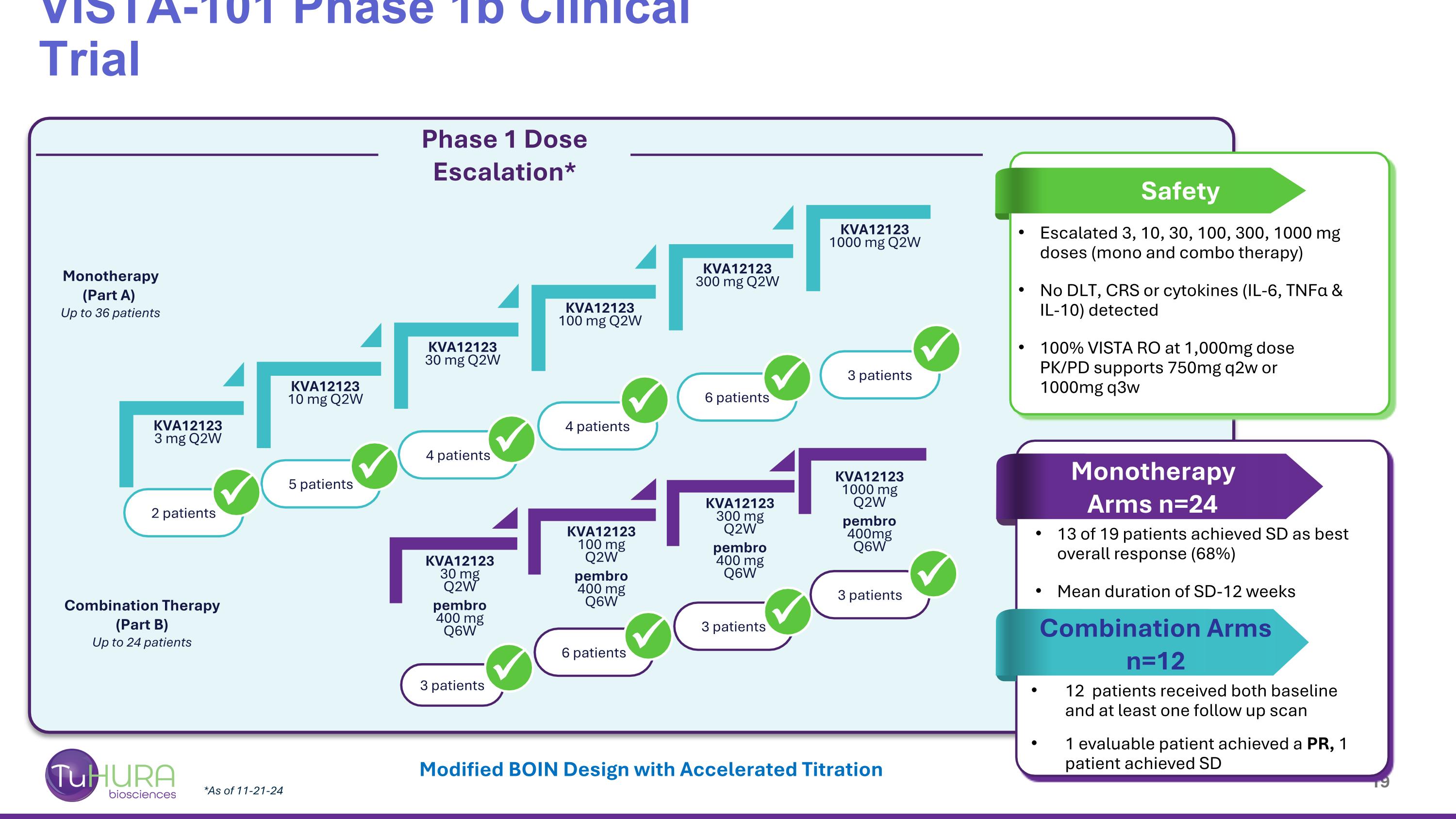
*As of 11-21-24 VISTA-101 Phase 1b Clinical Trial Modified BOIN Design with Accelerated Titration 3 patients KVA12123 3 mg Q2W KVA12123 10 mg Q2W KVA12123 30 mg Q2W KVA12123 100 mg Q2W KVA12123 300 mg Q2W KVA12123 1000 mg Q2W 2 patients 5 patients 4 patients 6 patients 4 patients 6 patients Monotherapy (Part A) Up to 36 patients Combination Therapy (Part B) Up to 24 patients KVA12123 30 mg Q2W pembro 400 mg Q6W KVA12123 100 mg Q2W pembro 400 mg Q6W KVA12123 300 mg Q2W pembro 400 mg Q6W KVA12123 1000 mg Q2W pembro 400mg Q6W 3 patients 3 patients Phase 1 Dose Escalation* Escalated 3, 10, 30, 100, 300, 1000 mg doses (mono and combo therapy) No DLT, CRS or cytokines (IL-6, TNFα & IL-10) detected 100% VISTA RO at 1,000mg dose PK/PD supports 750mg q2w or 1000mg q3w 13 of 19 patients achieved SD as best overall response (68%) Mean duration of SD-12 weeks 12 patients received both baseline and at least one follow up scan 1 evaluable patient achieved a PR, 1 patient achieved SD 3 patients Monotherapy Arms n=24 Safety Combination Arms n=12
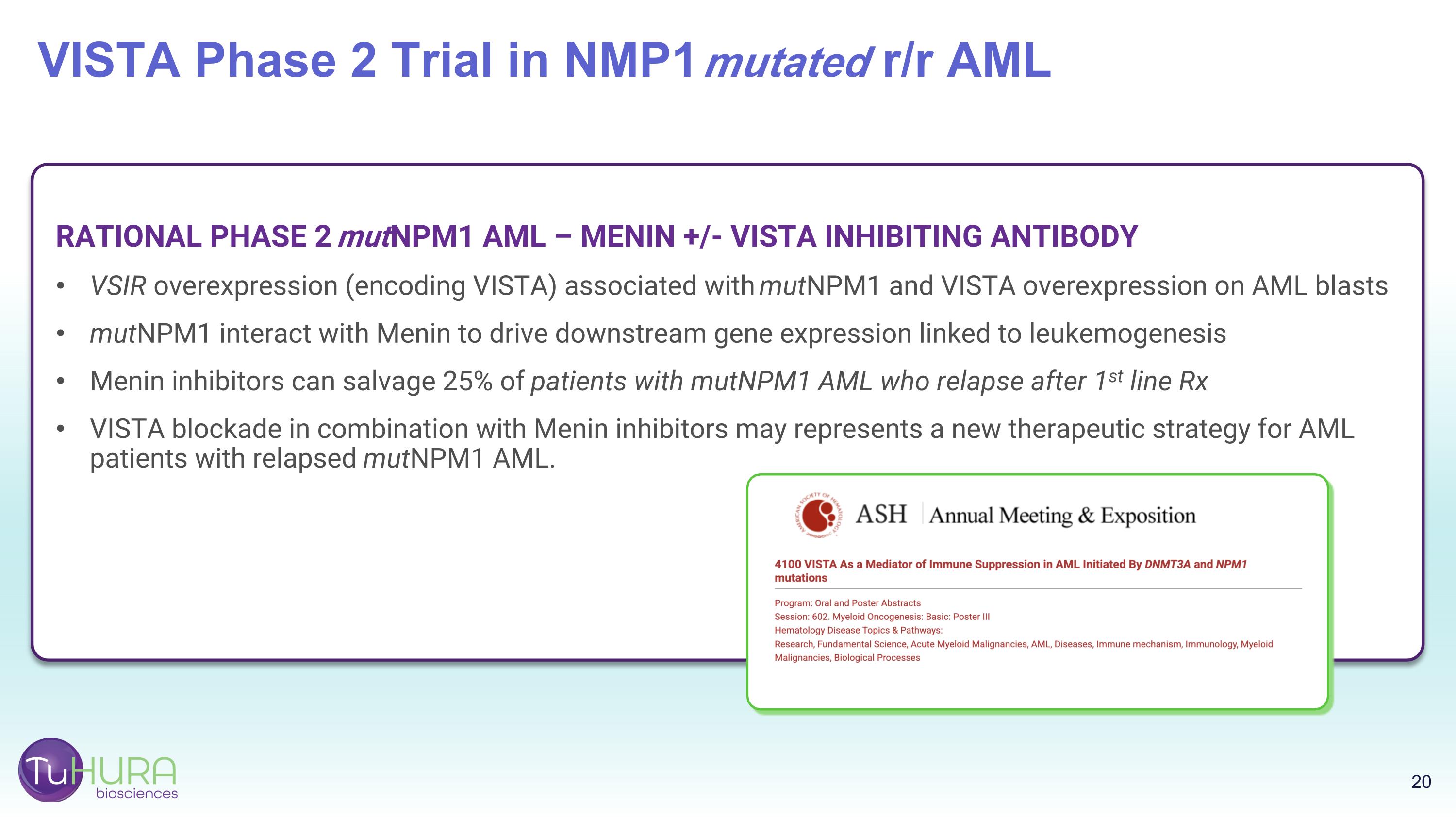
RATIONAL PHASE 2 mutNPM1 AML – MENIN +/- VISTA INHIBITING ANTIBODY VSIR overexpression (encoding VISTA) associated with mutNPM1 and VISTA overexpression on AML blasts mutNPM1 interact with Menin to drive downstream gene expression linked to leukemogenesis Menin inhibitors can salvage 25% of patients with mutNPM1 AML who relapse after 1st line Rx VISTA blockade in combination with Menin inhibitors may represents a new therapeutic strategy for AML patients with relapsed mutNPM1 AML. VISTA Phase 2 Trial in NMP1 mutated r/r AML
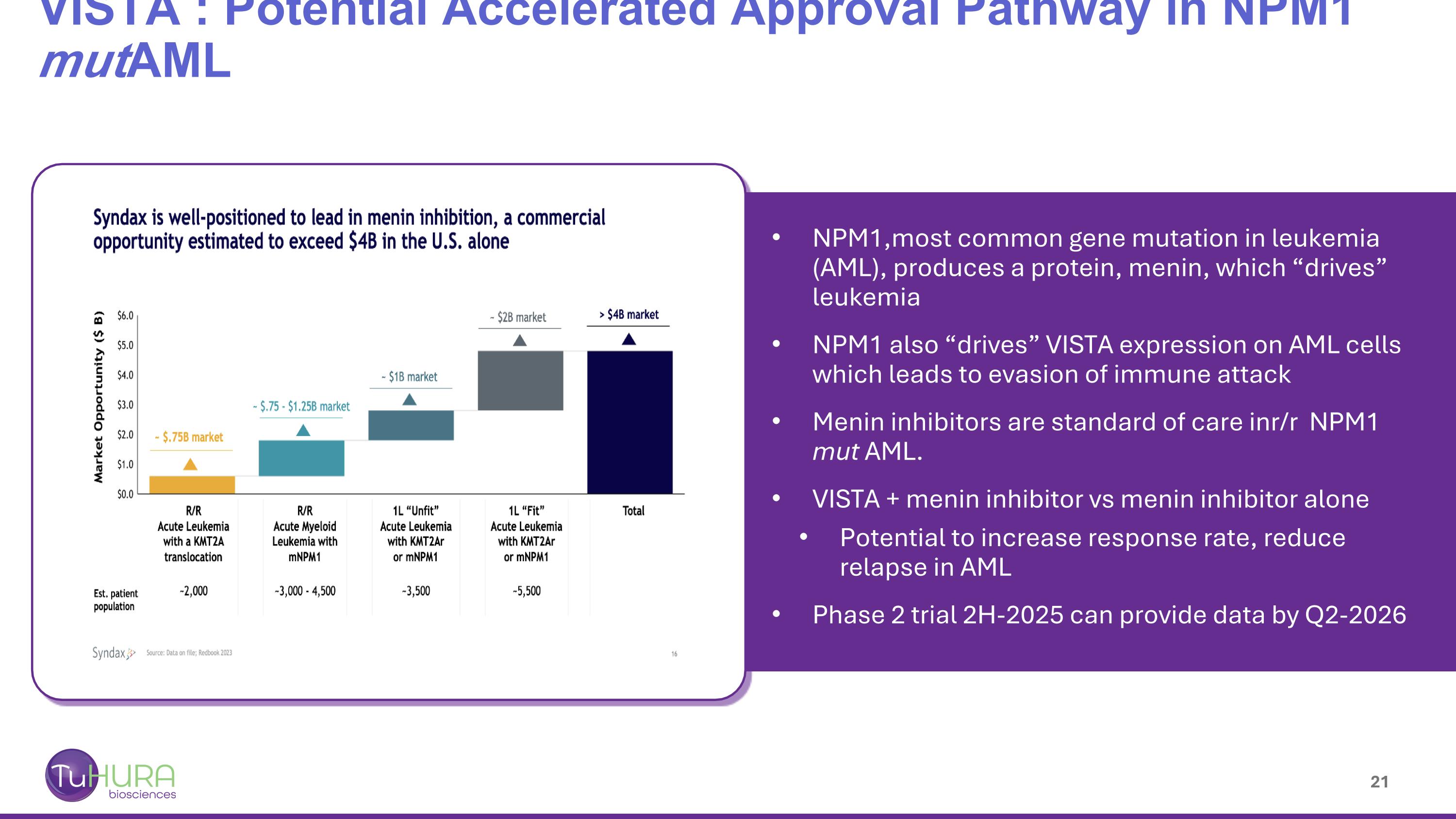
VISTA : Potential Accelerated Approval Pathway in NPM1 mutAML NPM1,most common gene mutation in leukemia (AML), produces a protein, menin, which “drives” leukemia NPM1 also “drives” VISTA expression on AML cells which leads to evasion of immune attack Menin inhibitors are standard of care inr/r NPM1 mut AML. VISTA + menin inhibitor vs menin inhibitor alone Potential to increase response rate, reduce relapse in AML Phase 2 trial 2H-2025 can provide data by Q2-2026

Tumor Microenvironment Modulators Novel Targets for Intervention Negative Immune Regulators (VISTA) MDSCs and M2 Macrophages (DOR inhibitors*) *DOR – Delta Opioid Receptor
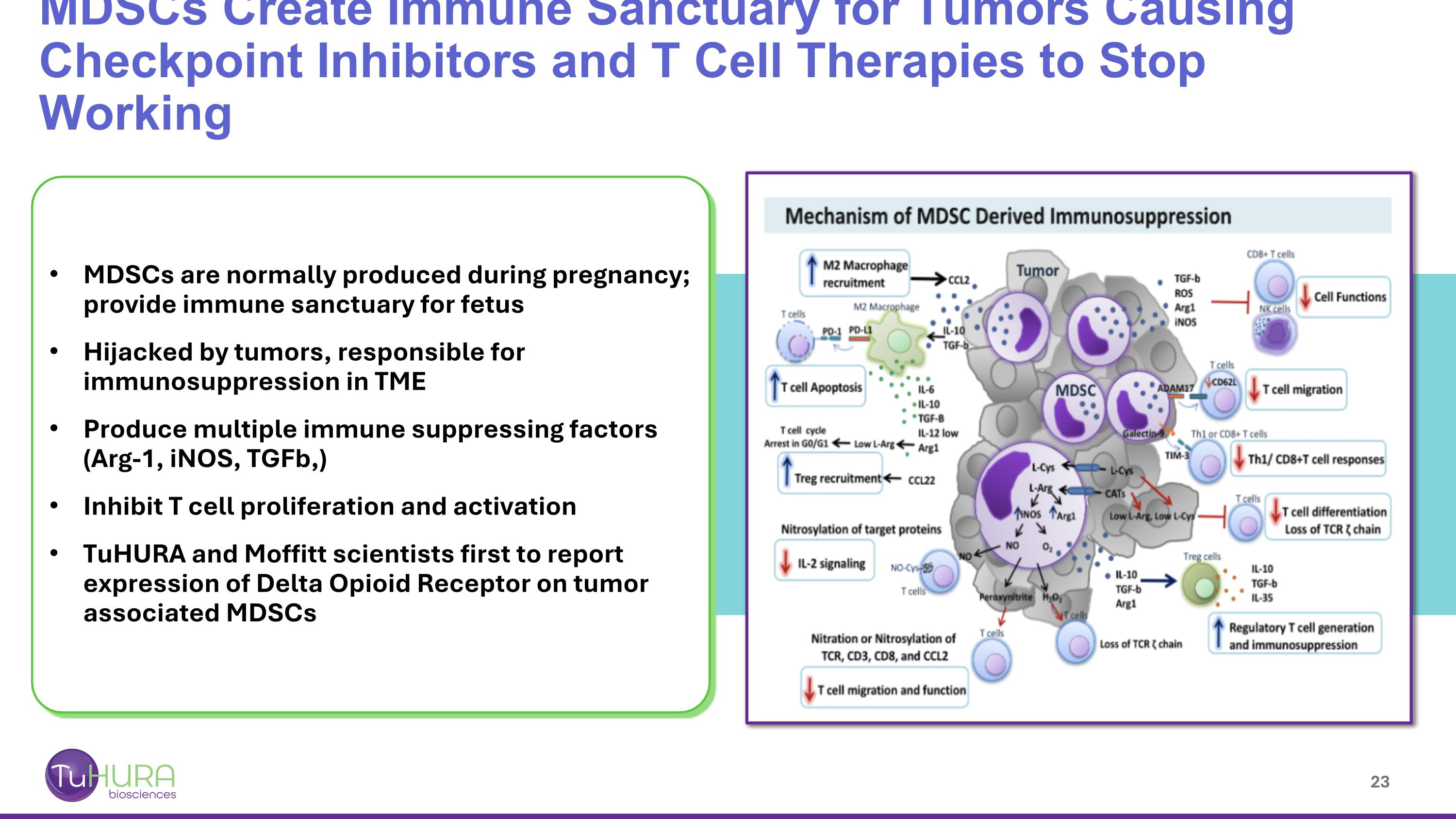
MDSCs are normally produced during pregnancy; provide immune sanctuary for fetus Hijacked by tumors, responsible for immunosuppression in TME Produce multiple immune suppressing factors (Arg-1, iNOS, TGFb,) Inhibit T cell proliferation and activation TuHURA and Moffitt scientists first to report expression of Delta Opioid Receptor on tumor associated MDSCs MDSCs Create Immune Sanctuary for Tumors Causing Checkpoint Inhibitors and T Cell Therapies to Stop Working
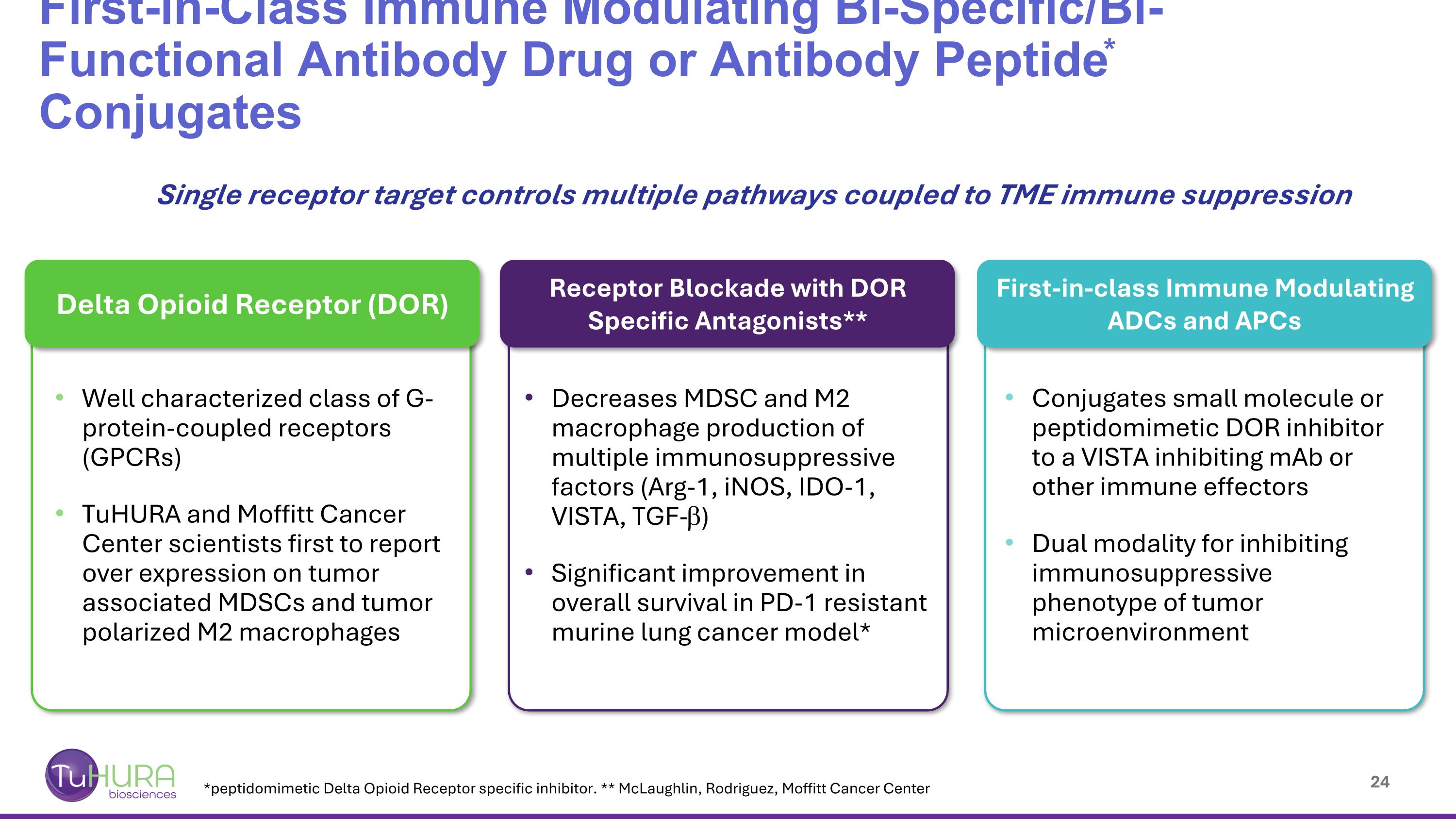
Delta Opioid Receptor (DOR) Well characterized class of G-protein-coupled receptors (GPCRs) TuHURA and Moffitt Cancer Center scientists first to report over expression on tumor associated MDSCs and tumor polarized M2 macrophages Decreases MDSC and M2 macrophage production of multiple immunosuppressive factors (Arg-1, iNOS, IDO-1, VISTA, TGF-b) Significant improvement in overall survival in PD-1 resistant murine lung cancer model* Receptor Blockade with DOR Specific Antagonists** Conjugates small molecule or peptidomimetic DOR inhibitor to a VISTA inhibiting mAb or other immune effectors Dual modality for inhibiting immunosuppressive phenotype of tumor microenvironment First-in-class Immune Modulating ADCs and APCs First-in-Class Immune Modulating Bi-Specific/Bi-Functional Antibody Drug or Antibody Peptide* Conjugates *peptidomimetic Delta Opioid Receptor specific inhibitor. ** McLaughlin, Rodriguez, Moffitt Cancer Center Single receptor target controls multiple pathways coupled to TME immune suppression

Corporate Highlights
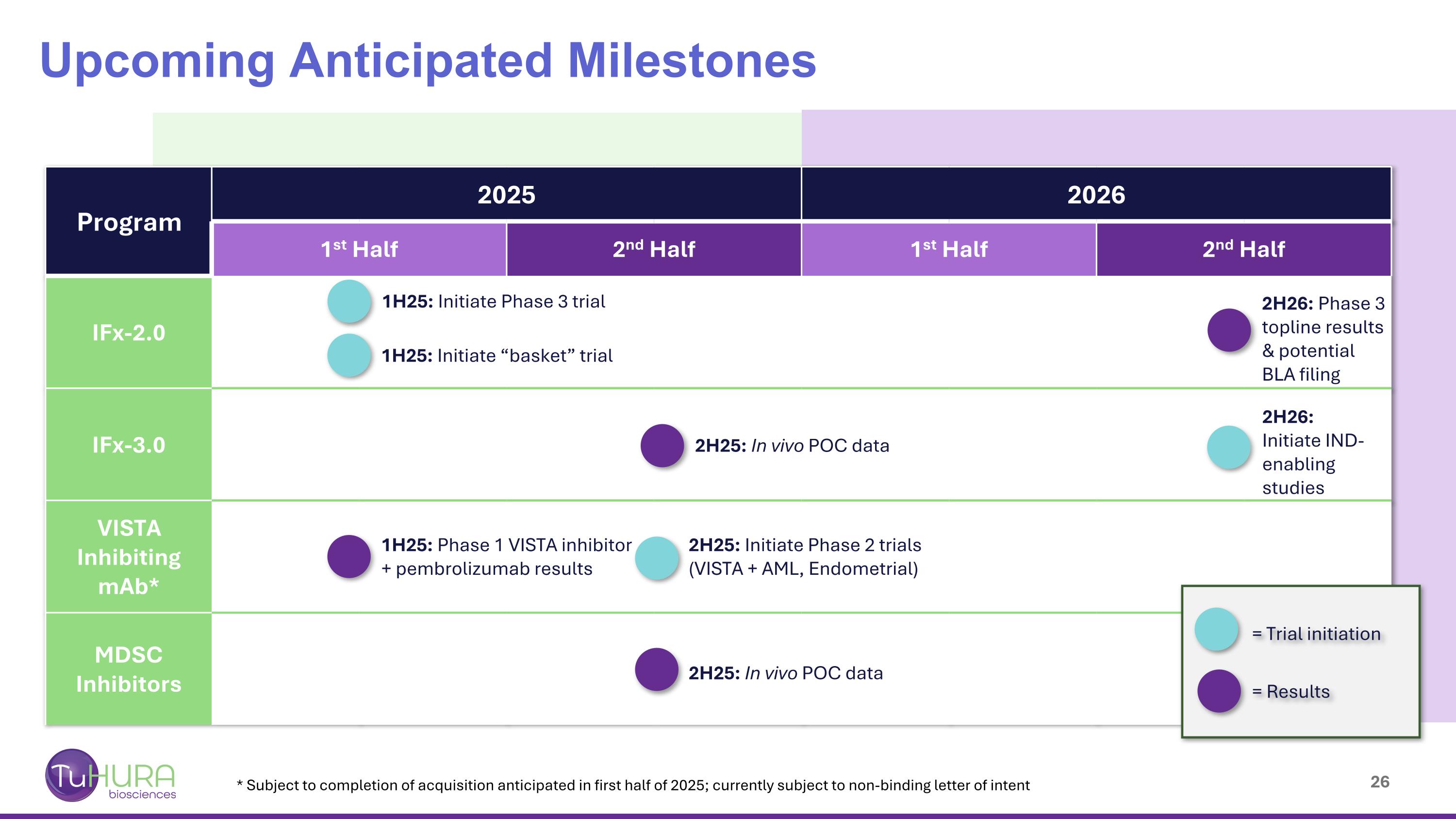
Program 2025 2026 1st Half 2nd Half 1st Half 2nd Half IFx-2.0 IFx-3.0 VISTA Inhibiting mAb* MDSC Inhibitors Upcoming Anticipated Milestones 1H25: Initiate Phase 3 trial 1H25: Phase 1 VISTA inhibitor + pembrolizumab results 1H25: Initiate “basket” trial 2H25: Initiate Phase 2 trials (VISTA + AML, Endometrial) 2H25: In vivo POC data 2H26: Initiate IND-enabling studies 2H25: In vivo POC data 2H26: Phase 3 topline results & potential BLA filing = Trial initiation = Results * Subject to completion of acquisition anticipated in first half of 2025; currently subject to non-binding letter of intent
Investment Summary * Trial currently subject to partial clinical hold relating to completion of certain CMC requirements for initiation of Phase 3 registration trial MCC = Merkel cell carcinoma Business Highlights Tumor Microenvironment Modulators Innate Immune Agonists Single Phase 3 registration directed Accelerated Approval trial for IFx-2.0* Demonstrated durable CRs and PRs in patients progressing on CPI in Phase 2 trial FDA Project Front Runner initiative encouraged trial in first line setting SPA Agreement with FDA on novel trial design could remove requirement for post approval confirmatory trial, potentially converting accelerated approval to full approval Top-line data anticipated 2H 2026 Building a de-risked late-stage product pipeline Definitive agreement to acquire by merger Kineta’s VISTA inhibiting mAb in clinical partnership with Merck If completed, Kineta transaction adds Phase 2 stage candidate to development pipeline in NMP1 mutated AML First-in-class, non-tumor targeting, bi-specific immune modulating ADCs/APCs Clinical, corporate and regulatory milestones with 4 key data readouts expected over the next 24 months
Overcoming Resistance to Cancer Immunotherapy tuhurabio.com