UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the fiscal year ended
or
Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from to
Commission File Number:
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
(Address of principal executive offices and Zip Code)
(
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
The |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
|
Accelerated Filer |
☐ |
☒ |
|
Smaller reporting company |
||
|
|
|
Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act) Yes ☐ No
The aggregate market value of the common equity held by non-affiliates of the registrant, based on the closing price of the shares of common stock on The Nasdaq Stock Market (“Nasdaq”) on June 30, 2024 (the last business day of the registrant’s second fiscal quarter), was $
Table of Contents
Forward-Looking Statements
i
EXPLANATORY NOTE
On October 18, 2024, the Nevada corporation formerly known as “Kintara Therapeutics, Inc.” completed its previously announced merger transaction in accordance with the terms of the Agreement and Plan of Merger, dated as of April 2, 2024 (the “Kintara Merger Agreement”), by and among Kintara Therapeutics, Inc. (“Kintara”), TuHURA Biosciences, Inc. (“Legacy TuHURA”), and Kayak Mergeco, Inc., a direct wholly owned subsidiary of Kintara (“Merger Sub”), pursuant to which Merger Sub merged with and into Legacy TuHURA, with Legacy TuHURA surviving as a direct wholly owned subsidiary of Kintara and the surviving corporation of the merger (the “Kintara Merger”). Additionally, as a result of the Kintara Merger, Kintara changed its name from “Kintara Therapeutics, Inc.” to “TuHURA Biosciences, Inc.” Following the completion of the Kintara Merger, the business of Legacy TuHURA became the primary business of the combined company, which is a clinical stage immune-oncology company developing novel personalized cancer vaccine product candidates designed to overcome resistance to immunotherapies like checkpoint inhibitors. Unless the context otherwise requires, “we,” “us,” “our,” and the “Company” refer to TuHURA Biosciences, Inc., a Nevada corporation, and its wholly owned subsidiaries (including Legacy TuHURA).
On October 18, 2023, in connection with the transactions contemplated by the Kintara Merger Agreement, Kintara filed a Certificate of Amendment to its articles of incorporation, as amended, effecting a 1-for-35 reverse stock split of Kintara’s common stock (the “Reverse Stock Split”). As a result of the Reverse Stock Split, the number of issued and outstanding shares of Kintara’s common stock immediately prior to the Reverse Stock Split was reduced such that every 35 shares of Kintara’s common stock held by a stockholder immediately prior to the Reverse Stock Split were combined and reclassified into one share of common stock after the Reverse Stock Split. Unless otherwise noted, all references to shares of common stock and per share amounts in this Annual Report on Form 10-K have been retroactively adjusted to reflect the shares issued to Legacy TuHURA stockholders in the Merger based on an exchange ratio of 0.1789 (the “Exchange Ratio”).
Under the terms of the Kintara Merger Agreement, immediately prior to the effective time of the Kintara Merger, shares of Legacy TuHURA’s preferred stock were converted into shares of Legacy TuHURA’s common stock and all of the convertible notes issued in Legacy TuHURA’s private placement financings were converted into shares of Legacy TuHURA common stock pursuant to the terms therein. At the effective time of the Kintara Merger, Kintara issued an aggregate of approximately 40,411,605 shares of its common stock to Legacy TuHURA stockholders, based on the Exchange Ratio. The issuance of the shares of Kintara’s common stock to the Legacy TuHURA stockholders was registered with the SEC on Kintara’s Registration Statement on Form S-4 (File No. 333-279368), as amended.
This Annual Report contains references to trademarks belonging to other entities, which are the property of their respective holders. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
1
FORWARD-LOOKING STATEMENTS
This annual report on Form 10-K (the “Annual Report”) contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 under Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”), as amended. Forward-looking statements include statements with respect to our beliefs, plans, objectives, goals, expectations, anticipations, assumptions, estimates, intentions and future performance, and involve known and unknown risks, uncertainties and other factors, which may be beyond our control, and which may cause our actual results, performance or achievements to be materially different from future results, performance or achievements expressed or implied by such forward-looking statements. All statements other than statements of historical fact are statements that could be forward-looking statements. You can identify these forward-looking statements through our use of words such as “may,” “can,” “anticipate,” “assume,” “should,” “indicate,” “would,” “believe,” “contemplate,” “expect,” “seek,” “estimate,” “continue,” “plan,” “point to,” “project,” “predict,” “could,” “intend,” “target,” “potential” and other similar words and expressions of the future.
There are a number of important factors that could cause the actual results to differ materially from those expressed in any forward-looking statement made by us. These factors include, but are not limited to:
2
Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to us. Such beliefs and assumptions may or may not prove to be correct. Additionally, such forward-looking statements are subject to a number of known and unknown risks, uncertainties and assumptions, and actual results may differ materially from those expressed or implied in the forward-looking statements due to various factors, including, but not limited to, those identified in Part I, Item 1A. “Risk Factors” and Part II, Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this Annual Report.
Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties.
You should read this Annual Report and the documents that we reference in this Annual Report completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements. Except as required by applicable law, we have no obligation to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
3
PART I
Item 1. Business
Overview
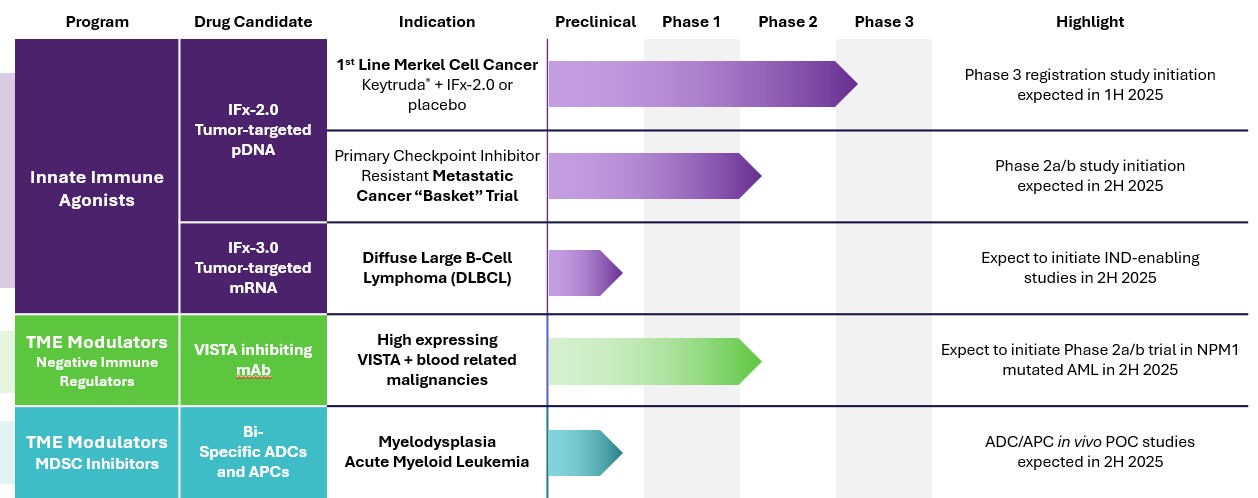
We are a clinical stage immuno-oncology company developing novel technologies designed to overcome primary and acquired resistance to cancer immunotherapies. Our lead product candidate, IFx2.0, is an innate immune agonist designed to overcome primary resistance to checkpoint inhibitors. We are preparing to initiate a single randomized placebo-controlled Phase 3 registration trial of IFx-2.0 administered as an adjunctive therapy to Keytruda® (pembrolizumab) in first line treatment for patients with advanced or metastatic Merkel cell carcinoma who are checkpoint inhibitor naïve utilizing the FDA’s accelerated approval pathway. In addition to our innate immune agonist candidates, we are leveraging our Delta receptor technology to develop tumor microenvironment modulators in the form of first-in-class bi-specific antibody-peptide conjugates (“APCs”) and antibody-drug conjugates (“ADCs”) targeting Myeloid Derived Suppressor Cells (“MDSCs”). Our APCs and ADCs are being developed to inhibit the immune-suppressing effects of MDSCs on the tumor microenvironment to prevent T cell exhaustion and acquired resistance to checkpoint inhibitors and cellular therapies.
Recent Developments
We are a Nevada corporation formed on June 24, 2009 under the name Berry Only Inc. On January 25, 2013, we entered into and closed an exchange agreement, with Del Mar Pharmaceuticals (BC) Ltd. (“Del Mar (BC)”), 0959454 B.C. Ltd. (“Callco”), and 0959456 B.C. Ltd. (“Exchangeco”) and the security holders of Del Mar (BC). Upon completion of the exchange agreement, Del Mar (BC) became our wholly-owned subsidiary. On August 19, 2020, we completed a merger with Adgero Biopharmaceuticals Holdings, Inc., a Delaware corporation (“Adgero”), in which Adgero continued its existence under Delaware law and became our direct, wholly-owned subsidiary. Following the completion of the merger, we changed our name from Del Mar Pharmaceuticals, Inc. to Kintara Therapeutics, Inc. and began trading on Nasdaq under the symbol “KTRA.”
On October 18, 2024, Kintara completed a reverse merger transaction contemplated by the Kintara Merger Agreement. Pursuant to the Kintara Merger Agreement, Merger Sub merged with and into Legacy TuHURA, with Legacy TuHURA surviving the Kintara Merger and becoming our direct, wholly-owned subsidiary. In connection with the completion of the Kintara Merger, effective at 12:01 a.m. Eastern Time on October 18, 2024, Kintara effected a 1-for-35 reverse stock split of its common stock. Effective at 12:03 a.m. Eastern Time on October 18, 2024, the merger was completed, and effective at 12:04 a.m. Eastern Time on October 18, 2024, Kintara Therapeutics, Inc. was renamed “TuHURA Biosciences, Inc.”
On December 12, 2024, we announced that we had entered into a definitive merger agreement to acquire Kineta, Inc. (“Kineta”), a Delaware corporation that is developing KVA12123, an anti-VISTA antibody checkpoint inhibitor designed to reverse VISTA immune suppression and remodel the tumor microenvironment to overcome acquired resistance to immunotherapies. The merger agreement (the “Kineta Merger Agreement”) was entered into among our company, Kineta, Hura Merger Sub I, Inc., a Delaware corporation and direct wholly owned subsidiary of ours (“Hura Merger Sub I”), Hura Merger Sub II, LLC, a Delaware limited liability company and direct wholly owned subsidiary of ours (“Hura Merger Sub II”), and Craig Philips, solely in his capacity as the representative, agent and attorney-in-fact of the stockholders of Kineta. The Kineta Merger contemplates the acquisition of Kineta through a merger transaction pursuant to which Hura Merger Sub I will (a) merge with and into Kineta (the “First Merger”), with Kineta being the surviving corporation of the First Merger (also known as the “Surviving Entity”) and (b) immediately following the First Merger and as part of the same overall transaction as the First Merger, the Surviving Entity will merge with and into Hura Merger Sub II (the “Second Merger”, and together with the First Merger, the “Kineta Merger”), with Hura Merger Sub II being the surviving company of the Second Merger.
At the effective time of the First Merger, and subject to the terms and conditions of the Kineta Merger Agreement, each share of Kineta’s common stock, par value $0.001 per share (“Kineta Common Stock”) issued and outstanding immediately prior to the effective time of the First Merger will be converted automatically into and will represent the right to receive, without interest, the number of shares of our common stock and cash consideration each calculated according to the terms of the Kineta Merger Agreement. The proposed Kineta Merger is expected to be consummated in the second quarter of 2025, subject to the satisfaction or waiver of closing conditions (including a financing condition) under the Kineta Merger Agreement.
4
IFx Innate Immune Agonist
We have developed Immune FxTM, or IFx, as an innate immune agonist technology designed to “trick” the body’s immune system to attack tumor cells by making tumor cells look like bacteria and to thereby harness the natural power of innate immunity by leveraging natural mechanisms conserved throughout evolution to recognize threats from foreign pathogens like bacteria or viruses. Our innate immune agonist product candidates are delivered either via intratumoral injection (in the case of the Company’s pDNA innate immune agonist) or tumor-targeted via intravenous or autologous whole-cell administration (in the case of our mRNA innate immune agonist).
Our IFx-2.0 innate immune agonist, our lead product candidate, is comparatively simple to administer and involves only the injection into a patient’s tumor, or lymph node, of a relatively small amount of pDNA that is designed to encode for an immunogenic gram positive bacterial protein that gets expressed on the surface of the patient’s tumor so that the surface of the tumor looks like a bacterium.
Bacteria, like all pathogens, have molecular patterns or motifs that are conserved through evolution and that are recognized by specific pattern-recognition receptors on immune cells of our innate immune system. This is an individual’s primary line of defense against pathogens that the individual is born with, and the innate immune system has no choice but to recognize the tumor as it would a gram-positive bacteria or any pathogen. Gram-positive bacterial proteins are recognized by Toll Like Receptor-2 (TLR-2) on antigen presenting cells, which engulf and ingest the entire intact tumor cell packaging all the foreign tumor neoantigens presenting them to and educating tumor killing T cells and B cells. In doing so, IFx-2.0 harnesses the power of the innate immune response to produce activated tumor-specific T cells where they previously didn’t exist overcoming primary resistance to checkpoint inhibitor therapy.
We have entered into a Special Protocol Assessment agreement with the FDA for a single Phase 3 randomized placebo and injection -ontrolled trial for IFx-2.0, our lead innate immune agonist, as an adjunctive therapy to pembrolizumab (Keytruda®) in the first line treatment of patients with advanced or metastatic Merkel cell carcinoma who are checkpoint inhibitor-naïve utilizing the FDA’s accelerated approval pathway. The Company worked with the deputy director of the FDA’s Oncology Center of Excellence (OCE) on a unique trial design. Consistent with the FDA’s Project Front Runner initiative, the FDA recommended investigating IFx-2.0 in the front-line treatment setting rather than in patients who are progressing on checkpoint inhibitor therapy. In doing so, data from a primary endpoint of objective response rate, or ORR, that is of sufficient magnitude and duration and with a favorable risk/benefit profile could be sufficient to support accelerated approval. Furthermore, OCE requested that we consider incorporating a key secondary endpoint that is of clinical benefit, such that results from a key secondary endpoint of progression-free survival, or PFS, that is adequately powered with statistical assumptions in the statistical analysis plan provided to the FDA, if achieved without a detrimental effect on overall survival, or OS, could be adequate to support conversion to regular approval satisfying the requirement for a confirmatory trial. Notwithstanding the foregoing, the results of clinical trials are inherently uncertain, and the results of our planned Phase 3 clinical trial may fail to satisfy the ORR, PFS, and/or OS endpoints, and none of our prior clinical trials with respect to IFx-2.0 were powered to determine statistical significance over a control.
As set forth in a January 2024 partial clinical trial hold letter from the FDA regarding the chemistry, manufacturing, and controls (CMC) requirements for our planned Phase 3 trial for IFx-2.0 to be conducted under the Special Protocol Assessment agreement, the FDA is requiring that, prior to initiating the trial, we must qualify potency assay and the mixing process for IFx-2.0. We have reached agreement with FDA on the requirements for lifting the partial clinical hold and believe we will meet the requirements and consequently expect to receive a complete response letter, or CRL, lifting the partial clinical hold in the second quarter of 2025. We may be in position to initiate the Phase 3 study in the second quarter of 2025 if the results of the mixing studies and potency assay qualifications are acceptable to the FDA, but there is no assurance that we will be able to satisfy the requirements set forth in the partial clinical trial hold letter on a timely basis or at all. We anticipate that enrollment for the Phase 3 would take approximately 12 months, with top-line data potentially being available 6 to 7 months following the last patient enrolled. If successful, this Phase 3 trial would form the basis of a Biologics License Application, or BLA. A Special Protocol Assessment agreement is a binding written agreement between the U.S. Food and Drug Administration (FDA) and a trial sponsor that indicates the study’s design and analysis are adequate to support an application submission. A Special Protocol Assessment agreement does not increase the likelihood of marketing approval for the product and may not lead to a faster or less costly development, review, or approval process.
We are also developing our IFx-3.0 product candidate, an mRNA innate immune agonist candidate for intravenous or autologous whole cell administration for blood-related cancers, to expand the utility of our IFx™ technology to tumor types not accessible by intra-tumoral injection. We are designing our mRNA innate immune agonist to be carried by a targeted lipid nanoparticle (“LNP”) coupled to an antibody which is intended to recognize and target CD22, a receptor overexpressed on B cell cancers like lymphoma. We believe that our novel LNP-anti CD22 construct may be the first intravenously administered, tumor-targeted mRNA innate immune agonist in preclinical development. Subject to further testing and development, we believe that systemically targeting a tumor with our mRNA innate immune agonist should induce a more widespread innate immune response
5
given the larger tumor burden associated with blood-related malignancies than with localized injection into small cutaneous or other accessible lesions.
Tumor Microenvironment Modulators: Bi-Specific/Bi-Functional Antibody Peptide Conjugates (APCs) and Antibody Drug Conjugates (ADCs) using Delta Opioid Receptor Technology
In addition to our innate immune agonist product candidates, we are using proprietary Delta Opioid Receptor technology to develop small molecule or bi-specific/bi-functional immune modulating APCs and ADCs designed to inhibit the immune suppressing effects of tumor associated MDSCs on the tumor microenvironment, TME, to prevent T cell exhaustion and acquired resistance to checkpoint inhibitors. Our Delta Opioid Receptor technology was developed by scientists at Moffitt Cancer Center and TuHURA Biopharma, Inc., a separate company whose intellectual property assets we acquired in January 2023 (“TuHURA Biopharma”). We believe the Delta Opioid Receptor represents a novel target to inhibit the immunosuppressive capacity of MDSCs through its control of the production of multiple immunosuppressive soluble factors.
The tumor microenvironment is the tissue surrounding a tumor, including the normal cells, blood vessels, and molecules that surround and feed a tumor cell and shield it from immune attack and eradication. MDSCs are a heterogeneous group of immature myeloid cells, which when recruited from the bone marrow to the tumor microenvironment, they transform to tumor-associated MDSCs which are characterized by their ability to suppress both innate and adaptive immune responses. Tumor associated MDSCs are generally believed to be a major contributor to T cell exhaustion (which is the loss of ability of T cells to proliferate and to kill cancer cells) and for acquired resistance to checkpoint inhibitors and cellular therapies like T cell therapies. The presence of tumor associated MDSCs in the tumor microenvironment or circulating in the bloodstream is highly correlated with poor prognosis and outcome in a wide variety of solid tumors and blood related cancers.
We believe we are the first company developing immune modulating APC/ADCs targeting the Delta Opioid Receptor on MDSCs. We are developing peptidomimetic or small molecule DOR-selective inhibitors to incorporate into our bi-specific/bi-functional APCs and ADCs, which we believe represents a paradigm shift from conventional APCs or ADCs that are currently in development or being marketed. Traditional ADCs are a class of drugs in which a monoclonal antibody is chemically linked to a cancer-fighting substance. The antibody carries the cancer fighting payload to the tumor cell improving the selectivity of the resulting anti-cancer activity. Next generation ADCs incorporate non-chemotherapeutic technologies to interfere with tumor cell cycle growth or to carry with the antibody a checkpoint inhibitor (so called “checkpoint ADCs”). In contrast, our APCs or ADCs do not target tumor associated receptor targets but rather target the Delta Opioid Receptor on MDSCs while carrying with them an immune effector to target a second receptor target like VISTA with a VISTA inhibiting antibody or other checkpoint inhibitor(s) producing novel bi-specific conjugates. These[CC1] two functions are intended to work together with the goal of overcoming acquired resistance, preventing T cell exhaustion and allowing checkpoint inhibitors and cellular therapies to be safer and more effective while interfering with the tumor’s ability to invade and spread throughout the body.
These two functions are intended to work together with the goal of overcoming acquired resistance, preventing T cell exhaustion and allowing checkpoint inhibitors and cellular therapies to be safer and more effective while interfering with the tumor’s ability to invade and spread throughout the body.
Our Pipeline
Our pipeline focuses on acquiring and developing technologies designed to overcome tumor-intrinsic mechanisms underlying primary resistance to checkpoint inhibitors. We also focus on technologies to overcome acquired resistance to cancer immunotherapies related to the immune suppressing characteristics of the tumor microenvironment. We are leveraging our technology platforms to advance several diversified product candidates, including principally the following:
6

Fx-2.0 Innate Immune Agonist. IFx-2.0 is our lead product candidate. We received guidance from and worked with the FDA’s Office of Tissues and Advanced Therapies and Oncology Center of Excellence in developing the Phase 3 trial for IFx-2.0.
IFx-2.0 Phase 1b/2a Basket Trial. We are planning a Phase 1b/2a trial referred to as a “basket” trial, which is a type of clinical trial that tests a new product candidate in patients who have different types of cancer but common biologic reason for resistance to checkpoint inhibitors.
Approximately 30% to 35% of patients with advanced Merkel cell carcinoma do not present with cutaneous or nodal lesions and are therefore not eligible for participation in our Phase 3 study. In the first stage of this basket study, IFx-2.0 will be administered via interventional radiology into lesions in the liver, retroperitoneum or lungs of patients who have advanced and metastatic Merkel cell carcinoma who are checkpoint inhibitor naïve who accessible cutaneous or lymph nodes. This trial will has the potential to extend the application of IFx-2.0.
The Phase 2a stage of the trial will include patients with checkpoint inhibitor resistant cancers such as triple negative breast cancer or other cancers known not to respond to checkpoint inhibitor therapy. We currently anticipate initiating this study in second half of 2025. If successful, this trial could expand the utility of IFx-2.0 beyond advanced Merkel cell carcinoma.
IFx-3.0. IFx-3.0 is our mRNA innate immune agonist for intravenous or autologous whole cell administration. We believe that advancing an mRNA innate immune agonist candidate for systemic or autologous whole cell administration may allow our company to expand the utility of our innate immune agonist technology to blood-related cancers, which are not amenable to intratumoral administration. The first planned application of IFx-3.0, is to target the CD22 receptor, which is over expressed on a number of B cell cancers like aggressive lymphomas. We plan to test various constructs in vitro before advancing to humanized immune competent murine models of aggressive lymphoma planned for 2026.
Antibody drug or Peptide conjugates. We are also developing novel immune modulating bi-functional ADCs and APCs to modulate the tumor microenvironment by reprograming MDSCs’ immune suppressing capabilities through inhibition of the Delta Opioid Receptor on MDSCs while localizing checkpoint inhibitors to checkpoint release activated T cells in the tissue where the tumor resides. We are working on developing and expanding a portfolio of novel Delta Opioid Receptor specific small molecule or peptidomimetic inhibitors of MDSC immunosuppressive functions as potential modulators of the tumor microenvironment alone or conjugated to an immune effector to construct its bi-functional ADCs. The Company’s prototype APC consists of a proprietary peptidomimetic inhibitor of the Delta Opioid Receptor conjugated to an antiPD-1 checkpoint inhibitor. Preclinical studies demonstrated that our APC significantly prolonged survival compared to antiPD-1 checkpoint inhibitor alone in a murine model of PD-1 resistant lung cancer.
Our History and Team
Our predecessor company was formed as Morphogenesis, Inc. in 1995 by Drs. Patricia and Michael Lawman. Our IFx technology was developed in the laboratory of Dr. Michael Lawman at the Walt Disney Memorial Cancer Institute, where Dr. Michael Lawman was formerly a Director of the Institute, and Dr. Patricia Lawman was formerly Division Director of Cancer Molecular Biology at the Institute. Dr. Michael Lawman is a Fellow of the Royal Society of Biology, former Associate Professor at University of South Florida, and former Scientific Research Director of Pediatric Hematology/Oncology at St. Joseph’s Children’s Hospital. Dr.
7
Patricia Lawman also serves as an Adjunct Professor at University of South Florida. Drs. Patricia and Michael Lawman are each inventors on numerous U.S. and foreign patents.
With respect to our bi-functional ADC technology, our Delta receptor APC and ADC technology was developed in the laboratory of Dr. Mark McLaughlin at the Moffitt Cancer Center and at the West Virginia University Research Corporation. Dr. McLaughlin was previously a Senior Member of the Drug Discovery Department at the Moffitt Cancer Center and previously Professor of Medicinal Chemistry and Member WVU Cancer Institute, where his research focused on protein-protein interaction inhibitor design and molecular targeted immunotherapy. The discovery that the Delta receptor is highly expressed on MDSCs was jointly discovered by scientists at Moffitt Cancer Center and TuHURA Biopharma, a separate company whose intellectual property assets we acquired in January 2023.
Our CEO, Dr. James Bianco, is a 30-year veteran of the biopharmaceutical industry. Dr. Bianco is the principal founder of CTI Biopharma, where he served as its CEO from 1992 to October 2016. Dr. Bianco’s experience spans all aspects of drug development from phase I-IV clinical trials, regulatory approval, and pricing reimbursement to sales and marketing. He has extensive experience in financing, negotiating and execution of pharmaceutical development and commercial license agreements. During his tenure at CTI Biopharma, Dr. Bianco was responsible for strategic portfolio development and identifying, acquiring, licensing, purchasing, or acquiring through international merger and acquisition, five drug candidates, four of which have since been approved by the FDA and with three receiving accelerated or conditional regulatory approval in the U.S. and/or E.U. In 2013, Dr. Bianco led CTI Biopharma in the identification and negotiation of the asset purchase for VONJO® (pacritinib), a novel JAK2 selective tyrosine kinase inhibitor. He also led CTI Biopharma in the negotiation of the development and commercial license agreement with Baxalta. As CEO of CTI Biopharma, Dr. Bianco was also responsible for the PERSIST-2 Phase 3 trial design and conduct, the results of which served as the basis for the 2022 FDA accelerated approval of pacritinib and the subsequent acquisition of CTI Biopharma by SOBI for $1.75 billion
Our Strategy
Our goal is to become a leading immuno-oncology company by developing innate immune agonist candidates designed to harness the power of the innate immune system to overcome primary resistance to cancer immunotherapies, broadening the impact of therapies such as checkpoint inhibitors. We are also developing novel bi-functional ADCs and APCs to modulate the tumor microenvironment by reprograming MDSCs’ immune suppressing capabilities through inhibition of Delta Opioid Receptor on MDSCs to overcome acquired resistance to cancer immunotherapies.
Our strategy leverages our technologies and novel product candidates to overcome primary and acquired resistance to checkpoint inhibitors, molecularly modified immune therapies and cellular therapies. The key elements of this strategy include:
8
Cancer Immunotherapies
The Cancer-Immunity Cycle
For an anti-cancer immune response to lead to effective killing of cancer cells, a series of stepwise events must be initiated and allowed to proceed and expand iteratively. These steps, which are illustrated in the graphic below, are referred to as the “cancer-immunity cycle”. The human immune system is comprised of the innate immune system and adaptive immune system. The innate immune response, through evolution, has developed to protect us from our surrounding environment. It is the defense system with which we are born and serves as the body’s first defense mechanism against pathogens like bacteria or viruses and alerts the immune system to those threats. It works together with its complementary arm, the adaptive immune system, to address threats in the body, including cancer.
In the first step of the cycle, foreign proteins called “neoantigens” are created by cancer-related genes and are released and captured by dendritic cells (“DCs”) for processing. In order for this step to lead to a tumor killing T cell response, it must be accompanied by signals that specify immunity, or otherwise tolerance to the tumor antigens will be induced. Such immunogenic signals might include proinflammatory cytokines and factors released by dying tumor cells. During the next step, DCs present the captured neoantigens on MHCI and MHCII molecules to T cells, resulting in the priming and activation of tumor cell killing, or cytotoxic, T cell responses against these cancer-specific neoantigens, which are viewed as foreign. Finally, the activated cytotoxic T cells traffic to and infiltrate the tumor bed, specifically recognizing and binding to cancer cells through the interaction between its T cell receptor (“TCR”) and its cognate antigen bound to MHCI and kill their target cancer cell. Killing of the cancer cell releases additional tumor-associated neoantigens repeating the first step of the cancer- immunity cycle, to increase the breadth and depth of the response in subsequent revolutions of the cycle.
In cancer patients, the cancer-immunity cycle does not perform optimally. In order for an innate response to be activated against a tumor, the tumor must appear foreign to the immune system. Tumor neoantigens may not be detected due to low neoantigen load or mutational burden, DCs and T cells may treat antigens as self rather than foreign thereby creating T regulatory cell responses rather than cytotoxic responses, T cells may not properly home to tumors, may be inhibited from infiltrating the tumor, or, importantly, factors in the tumor microenvironment might suppress those effector T cells that are produced. The goal of cancer immunotherapy is to initiate and reinitiate a self-sustaining cycle of cancer immunity, enabling it to amplify and propagate.
9
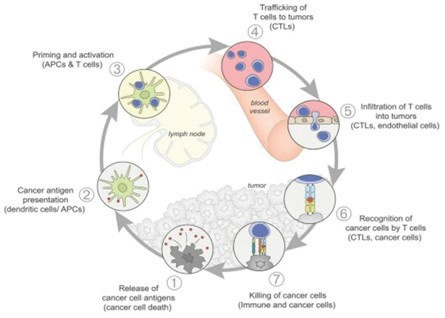
Source: Oncology Meets Immunology: The Cancer-Immunity Cycle, Immunity, Volume 39, July 2013
IFx Technology
The goal of cancer immunotherapies generally is to initiate an immune response to tumor neoantigens, which are the abnormal proteins that tumor-associated genetic mutations cause the cells to produce. There are a number of approaches that attempt to make a tumor look foreign to the immune system. The optimal cancer immunotherapy would make a patient’s entire tumor appear foreign and activate an innate immune response through the comprehensive and efficient packaging of tumor neoantigens which are presented to cytotoxic T cells, leading to their priming, activation, and proliferation of an immune attack against the tumor. Our IFx Technology is designed to accomplish this goal.
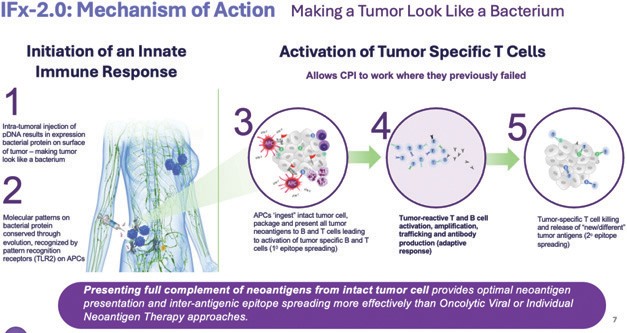
Our IFx platform technology utilizes a proprietary plasmid DNA (“pDNA”) or messenger RNA (“mRNA”) which, when introduced into a tumor cell, results in the expression of a highly immunogenic gram positive bacterial protein (Emm55) from a rare variant of Streptococcus pyogenes on the surface of the tumor cell. This is graphically demonstrated above. By mimicking a
10
bacterium, our technology makes a tumor cell look like bacteria. By making a tumor look like a bacterium, the molecular pattern of the bacterial protein is recognized by specific receptors on immune cells called pattern recognition receptors, also referred to as toll-like receptors or TLRs. These receptors are pre-programmed over evolution to recognize specific molecular patterns or motifs on pathogens like bacteria and activate and harness the power of the body’s innate immune response.
IFx is designed to harness the body’s natural innate immune response making the patients entire tumor appear foreign. This causes antigen presenting cells like DCs to phagocytize (which is the process of “eating” and “digesting”) the tumor cell, thinking they are bacteria. DCs present the captured neoantigens on MHCI and MHCII molecules to T cells, resulting in the priming and activation of cytotoxic T cell responses against these cancer-specific neoantigens, which are viewed as foreign. This is referred to as “primary epitope spreading.” Epitopes are the region/part of tumor antigens that are recognized by the immune system, specifically by antibodies, B cells and T cells. In doing so the first step of the cancer-immunity cycle is activated and restored.
Plasmid DNA, or plasmids, are small, circular, double-stranded DNA molecules that are separate from a cell’s chromosomal DNA and can replicate independently. Plasmids are most commonly found in bacteria, but can also be found in archaea and eukaryotic organisms. They can range in length from about 1,000 to hundreds of thousands of DNA base pairs. Plasmids often carry genes that can benefit the survival of an organism, such as antibiotic resistance. When a bacterium divides, all of the plasmids in the call are copied, so each daughter cell receives a copy of each plasmid. Plasmids can also be transmitted horizontally to other bacteria in some cases. Scientists have taken advantage of plasmids to use them as tools to clone, transfer, and manipulate genes.
Other Types of Cancer Immunotherapies
To date, most cancer immunotherapies, such as those described below, have utilized a number of different approaches to initiate an innate immune response to generate tumor specific activated T cells.
Oncolytic Virus Vaccines. Oncolytic virus vaccines are designed to preferentially induce viral replication-dependent oncolysis (viral induced killing) in tumors in an effort to stimulate antitumor immune responses. Intratumoral injection is thought to trigger both local and systemic immunological responses leading to tumor cell lysis, the release of tumor-associated antigens into the tumor microenvironment where they need to be recognized by antigen presenting cells leading to subsequent activation of innate and adaptive immune systems to induce tumor antigen-specific effector T-cell antitumor immunity.
Tumor-associated antigen vaccines. Another approach is to utilize Tumor-Associated Antigens (“TAAs”), some of which may also be similar to self-antigens, although preferentially overexpressed on tumor cells. However, these TAAs may also be displayed by normal healthy cells or cancer testis antigens that are only expressed by tumor cells and adult reproductive tissues. T and B cells with high affinity toward these TAAs also target self-antigens leading to the removal of these T and B cells from the immune repertoire by central and peripheral tolerance. Thus, a potent vaccine must break tolerance for them to work. To date, this approach has had limited success.
Individual Neoantigen Therapy. Tumor-Specific Antigens (“TSAs”) differ from tumor-associated antigens since they are not shared with similar self-antigens. They are typically de novo epitopes expressed by cancer-causing viruses (or oncoviruses) or private neoantigens encoded by somatic mutations. TSAs are truly tumor specific with no central tolerance. Deciding which TSAs to select and how to configure such multivalent vaccines is itself a daunting challenge. It may be insufficient to rely entirely on sequencing the expressed tumor genome looking for point mutations, translocation fusions, or CT antigens. Not only might this vary from patient to patient or even from cell to cell within a single patient’s tumor, expression at the messenger RNA or protein level does not assure that predicted antigenic peptides will be generated and expressed as peptide-MHCI complexes, especially in the face of the allelic complexity in the MHC. Several groups are actively approaching this problem by using a combination of informatics and mass spectroscopy of peptides eluted from MHCI molecules. Early clinical trials used as neo-adjuvant therapy in combination with checkpoint inhibitors among patients with potentially surgically curable disease at risk for relapse has yielded encouraging results, although how best to deliver them to patients remains a critical unknown.
Potential Advantages of IFx Innate Immune Agonist Technology
IFx’s approach is designed to naturally harness the power of the innate immune response leveraging Pathogen Associated Molecular Patterns (PAMP), or motifs present on pathogens, like bacteria and conserved through evolution. These patterns are recognized by pattern recognition receptors on antigen presenting and other immune cells of our innate immune system. By expressing a bacterial protein on the surface of a tumor cell the intact tumor cell is digested and the full complement of foreign tumor neoantigens are packaged and presented to newly produced T and B cells producing activated tumor specific T cells, the primary target allowing checkpoint inhibitors to work where they previously failed.
11
We believe that our IFx technology avoids problems associated with trying to predict which tumor- specific antigens are important and avoids the challenges associated with selection, analysis, production and delivery that accompanies individual neoantigen therapy approaches. Unlike oncolytic viral therapies which lyse the tumor cell disseminating tumor neoantigens throughout the tissue surrounding the tumor relying on antigen presenting cells in the vicinity to recognize, digest and present neoantigens to naïve T and B cells, IFx technology presents the full complement of tumor neoantigens from the intact tumor cell providing more optimal neoantigen presentation and inter-antigenic epitope spreading more effectively than oncolytic viral therapy or individual neoantigen therapy approaches.
Importantly, IFx is not an oncolytic viral technology. Oncolytic viral technologies which work by “exploding” the tumor cell resulting in the random dissemination of tumor neoantigens into the tumor microenvironment where immune cells can potentially see and digest them. In contrast, IFx presents the full complement of tumor neoantigens packaged inside the intact tumor cell providing much more optimal neoantigen presentation and more efficient inter-antigenic epitope spreading.
Bi-specific/Bi-functional APCs and ADCs: Inhibiting MDSC immune suppressing functions
MDSCs
MDSCs are among the most common cells present in the tumor microenvironment, which is the tissue surrounding the tumor, where they are a major regulator of suppression of the immune system. MDSCs are normally produced during pregnancy where they migrate to and populate the placenta, creating an immunologic sanctuary for the fetus. Since half of the genetic make-up of the fetus comes from the father, this is necessary to prevent the mother’s immune system from attacking the fetus. They are also produced in settings of chronic inflammation or autoimmune disease as a mechanism to decrease inflammation or autoimmunity. Under normal conditions, MDSCs represent less than 2% of circulating peripheral blood mononuclear cells (PBMCs) and lack potent immune suppressing characteristics
In cancer, MDSCs are hijacked by tumors to create an immunosuppressive environment in the tissues in which the tumor lives. MDSCs are the primary driver of the immunosuppressive tumor microenvironment. Multiple effector molecules and signaling pathways are used by MDSCs to regulate immune suppression. One main mechanism involves depletion of necessary amino acids like arginine through production of arginase (“Arg-1”), or “destruction” of inflammatory cytokines via production of inducible nitric oxide (“iNOS”), in addition to anti-inflammatory prostaglandins (“COX2”), immune suppressing cytokines like transforming growth factor beta (“TGF-®”) or Interleukin 10 (“IL-10”) and recruitment and induction of immune inhibitory cells such as regulatory T cells (T regs) and M2 polarized tumor associated macrophages (“TAMs”). Accumulating evidence demonstrates that the enrichment and activation of MDSCs correlates with tumor progression, metastasis and recurrence. In addition, MDSCs circulating in the blood of patients with cancer is highly correlated to poor clinical outcome.
12
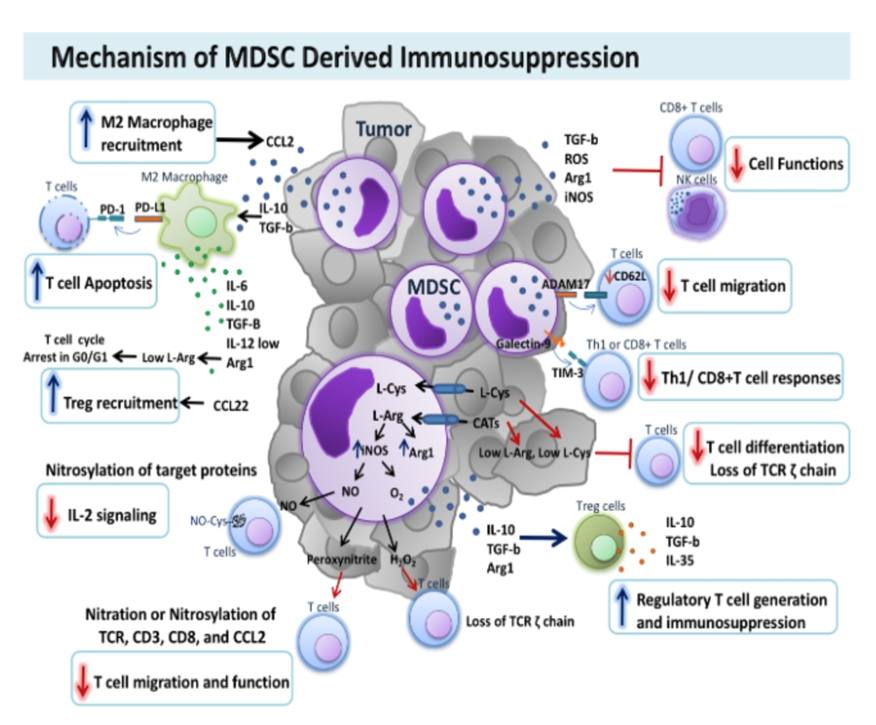
We believe that inhibiting and reprograming MDSC function represents a promising novel approach to overcome MDSC-induced tumor microenvironment immunosuppression and the resulting acquired resistance to cancer immunotherapies. Various companies are focusing on several strategies, including blocking MDSC recruitment to the microenvironment or inhibiting their production in the bone marrow. Another potential strategy is inhibiting MDSC-mediated immunosuppression by developing inhibitors to individual MDSC- related immune suppressing compounds such as IDO, iNOS or COX2 inhibitors.
Our Delta Opioid Receptor (DOR) inhibitors: bi-specific, bi-functional antibody peptide or drug conjugates (APC, ADCs)
The Delta Opioid Receptor, or DOR, is the first cloned G protein-coupled receptor. Many recent studies on Delta Opioid Receptor functions have determined that the Delta Opioid Receptor is involved in the regulation of malignant transformation and tumor progression in multiple cancers. In hepatocellular carcinoma (HCC), higher expression of Delta Opioid Receptor was observed in liver tumor tissue cells compared to normal liver tissue/cells. When Delta Opioid Receptorgene expression was silenced or inhibited, the proliferation of HCC ells was inhibited, and tumor cells underwent apoptosis, the cell cycle was arrested and tumor cell invasion and migration.
While Delta Opioid Receptor overexpression and its role in tumor biology is well established in the literature, we believe that the Company, along with scientists at Moffitt Cancer Center, are the first to describe the high differential expression of the Delta Opioid Receptor on tumor associated MDSCs compared to bone marrow (BM) or spleen derived MDSCs either in tumor free or tumor bearing models. (See Figures 1 and 2 below, courtesy of P Rodriguez, Moffitt Cancer Center ).
As a previously unrecognized target to reprogram tumor associated MDSCs immunosuppressive functions on the tumor microenvironment, developing small molecule or peptide antagonists of the Delta Opioid Receptor represents a novel approach to reprograming MDSC functionality to overcome acquired resistance to checkpoint inhibitors and other cancer immunotherapies.
13
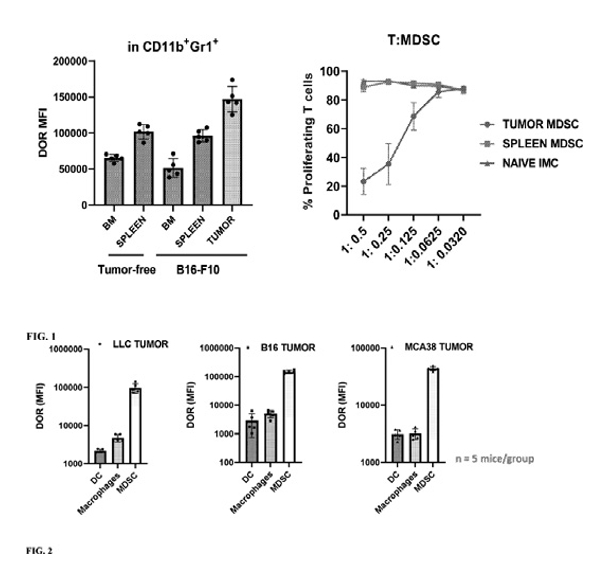
Inhibition of the Delta Opioid Receptor on tumor associated MDSCs is designed to block MDSC production of multiple immunosuppressing factors through a single point of intervention. Our bi-specific APCs consist of a patented peptidomimetic Delta Opioid Receptor specific inhibitor conjugated to a checkpoint inhibitor like anti-PD-1 antibody. Moffitt Cancer Center scientists demonstrated that in Delta Opioid Receptor expressing, PD-1 resistant murine lung cancer models, treatment with its APC, accumulated in the tumor microenvironment and resulted in a significant and dramatic improvement in survival when compared to treatment with anti-PD-1 antibody alone. We have established multiple functional assay screens to investigate the effects of both novel peptidomimetic or small molecule Delta Opioid Receptor specific inhibitors of tumor associated MDSC functionality to guide its selection of both APCs and ADCs for further in vitro and in vivo characterization and development.
We believe that our tumor associated MDSC-targeting APCs and ADCs have a number of potential benefits over current approaches to overcoming acquired resistance to cancer immunotherapies, including the following:
14
Our Clinical Development Program
For purposes of the below descriptions of our Phase 1 and 1b clinical trials, the response rates for IFx-2.0 are determined under best clinical practice by the principal investigators, evaluating and confirming clinical progression prior to or during therapy utilizing conventional and appropriate radiographic or metabolic (Positron Emission Tomography – PET) methodologies. Response determination utilizes conventional terminologies under standardized response evaluation criteria. A “complete response”, or CR, is deemed to be disappearance of all lesions. A “partial response”, or PR, is at least a 30% decrease in the sum of the size of the target lesions. “Progressive disease”, or PD, is at least a 20% increase in the sum of the longest diameter or the appearance of new lesions. “Stable disease”, or SD, means that the patient has neither sufficient shrinkage in the lesions to qualify for PR nor sufficient increase to qualify for PD. The term “objective response rate” is defined as the proportion of patients who have a partial or complete response to therapy. Furthermore, the term “pCR” refers to a pathological complete response, which is the absence of signs of cancer in tissue samples removed during surgery or biopsy after treatment. “Progression-free survival”, or PFS, means the length of time after the treatment that a patient lives without disease progression.
Planned Phase 3 Trial for IFx-2.0
We have entered into a Special Protocol Assessment agreement with the FDA for a single Phase 3 randomized placebo and injection controlled trial for IFx-2.0, our lead innate immune agonist, as adjunctive therapy to pembrolizumab (Keytruda®) in the first line treatment of patients with advanced or metastatic Merkel cell carcinoma who are checkpoint inhibitor-naïve utilizing the FDA’s accelerated approval pathway. The Company has worked the deputy director of the FDA’s Oncology Center of Excellence (OCE) on a unique trial design. Consistent with the FDA’s Project Front Runner initiative, the FDA recommended investigating IFx-2.0 in the front line treatment setting rather than in patients who are progressing on checkpoint inhibitor therapy, the latter of which was the conduct in the phase 1b trial. In doing so, data from a primary endpoint of objective response rate, or ORR, that is of sufficient magnitude and duration and with a favorable risk/benefit profile could be sufficient to support accelerated approval. Furthermore, OCE requested that we consider incorporating a key secondary endpoint that is of clinical benefit such that results from a key secondary endpoint of progression-free survival, or PFS, that is adequately powered with statistical assumptions in the statistical analysis plan provided to the FDA, if achieved without a detrimental effect on overall survival, or OS, could be adequate to support conversion to regular approval satisfying the requirement for a confirmatory trial. Notwithstanding the foregoing, the results of clinical trials are inherently uncertain, and the results of our planned Phase 3 clinical trial may fail to satisfy the ORR, PFS, and/or OS endpoints, and none of our prior clinical trials with respect to IFx-2.0 were powered to determine statistical significance over a control.
As set forth in a January 2024 partial clinical trial hold letter from the FDA regarding the chemistry, manufacturing, and controls (CMC) requirements for our planned Phase 3 trial for IFx-2.0 to be conducted under the Special Protocol Assessment agreement, the FDA is requiring that, prior to initiating the trial, we must qualify potency assay and the mixing process for IFx-2.0 to be used at the clinical site. We have reached agreement with FDA on the requirements for lifting the partial clinical hold and believe we will meet the requirements and consequently expect to receive a complete response letter, or CRL, lifting the partial clinical hold in the second quarter of 2025. The Company currently believes it may be in position to initiate the Phase 3 study in the second quarter of 2025 if the results of the mixing studies and potency assay qualifications are acceptable to the FDA, but there is no assurance that
15
we will be able to satisfy the requirements set forth in the partial clinical trial hold letter on a timely basis or at all. We anticipate that enrollment for the Phase 3 would take approximately 12 months, with topline data potentially being available 6 to 7 months following the last patient enrolled. If successful, this Phase 3 trial would form the basis of a Biologics License Application, or BLA. A Special Protocol Assessment agreement is a binding written agreement between the U.S. Food and Drug Administration (FDA) and a trial sponsor that indicates the study’s design and analysis are adequate to support an application submission. A Special Protocol Assessment agreement does not increase the likelihood of marketing approval for the product and may not lead to a faster or less costly development, review, or approval process. The study population, dose, schedule, and study design for the trial are based on the response rates observed in our Phase 1b trial in checkpoint inhibitor naïve patients with advanced Merkel cell carcinoma who exhibited primary resistance to anti PD(L)-1 checkpoint inhibitors such as Keytruda® The clinical study design for the Phase 3 registration trial is presented below. Based on correspondence with the FDA, patients with advanced Merkel cell carcinoma represent a patient population with an unmet medical need. Our study is designed to determine if IFx-2.0 can increase the objective response rate when used as adjunctive therapy to Keytruda in first line treatment of checkpoint inhibitor naïve patients with advanced Merkel cell carcinoma when compared to Keytruda alone.
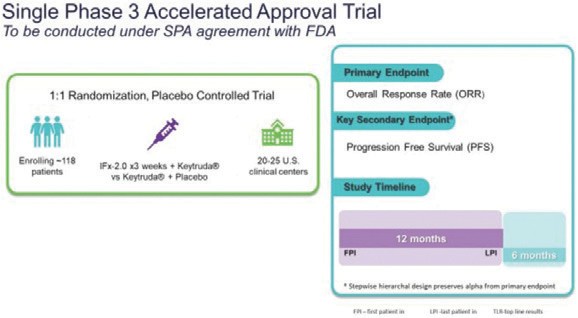
Note: “FPI” means first patient in, “LPI” means last patient in, and “TLR” means top-line results. Progression Free Survival, or PFS, is defined as the time from randomization until first evidence of disease progression or death, and Overall Survival, or OS, is defined as the time between randomization to death.
Phase 1b Trial in Metastatic Merkel Cell Carcinoma and Cutaneous Squamous Cell Carcinoma
We have completed enrollment in a multicenter Phase 1b dose and schedule finding trial for our IFx-Hu2.0 innate immune agonist candidate in patients with advanced Merkel cell carcinoma (MMC) or cutaneous Squamous cell carcinoma (cSCC). This study follows a two-stage design with a primary goal to assess the safety and feasibility of repeated dosing schemas of IFx-2.0. In the first stage (exposure escalation), a 3+3 trial design was utilized to assess safety of repeated weekly intratumoral injections using a fixed dose of IFx-2.0 weekly for 1, 2 or 3 weeks (for cohorts 1, 2 or 3 respectively). Following safety evaluation the protocol was amended to include an expansion stage to increase the total study sample size to 20. A total of 23 patients were enrolled. As of June 2024, follow-up data was available on all evaluable patients.
The primary objective of the trial was to determine the safety, tolerability, and optimal dose and schedule of IFx-2.0 when administered intratumoral in up to three lesions injected across three different administration schedules. Safety was evaluated for up to 28 days following IFx-2.0 administration. Secondary objectives include tumor shrinkage (injected and non-injected lesions) and
16
correlative immune response analysis (transcriptomic, proteomic, humoral and cellular), pre-and post-IFx-2.0 administration to guide the choice of dose and schedule for our Phase 3 registration directed trial.
Twenty-three (23) patients were enrolled: Merkel cell carcinoma (13), cSCC (10). Among the thirteen (13) patients with Merkel cell carcinoma, twelve (12) completed treatment and the protocol directed 28 day safety evaluation follow up period; One (1) patient experienced a serious adverse event, or SAE, deemed possibly related to study drug. This patient experienced a Grade 3, or G3, adverse event, which is defined as an adverse event that is a severe or medically significant event that is not immediately life threatening, which in the case of this patient was a G3 autoimmune hepatitis that resolved with steroid treatment, and such patent has been recently treated with checkpoint inhibitors prior to study enrollment. Among the ten (10) patients with cSCC one (1) patient experienced an SAE unrelated to study drug and did not complete treatment nor the 28 day safety evaluation follow up period. All patients had received prior anti-PD(L)1 based treatment with disease progression being the reason for CPI discontinuation in all patients but one. Intra- tumoral (IT) IFx-2.0 was well tolerated at all dose schedules evaluated. As to efficacy, in the 21 patients that completed the study, best overall disease response to trial therapy was PR in 1 patient (including both injected and non-injected tumor sites), SD in 4, and PD in 16. The response assessment limited to the injected site(s) only was PR in 2 patients, SD in 8, and PD in 9. Two additional patients were not evaluable at the injected site(s) due to clinically challenging to measure dermal lesions that were not radiographically measurable. The study achieved the primary safety endpoint of the study demonstrating no grade 3 or greater toxicity in any of the 3 dose levels examined, and as a result, a recommended phase 2 dose was determined. The study also achieved its secondary endpoint of efficacy analysis demonstrating a disease control rate of 48% among injected lesions within the first 28 days post injection, and, as described below, a post-protocol efficacy analysis demonstrated an overall objective response rate of 64% (7 of 11 patients with Merkel cell carcinoma) after re-challenge with immune checkpoint inhibitors.
After protocol specified IT therapy, eleven (11) Merkel cell carcinoma patients and six (6) cSCC pts were treated with anti-PD(L)1 based therapy as the immediate post-protocol treatment. Five (5) of nine (9) (56%) evaluable Merkel cell carcinoma patients and one (1) of (6) (17%) cSCC patients experienced an objective response to this ICI rechallenge, with duration of response ongoing in four (4) patients (6+, 19+, 21+, 23+ months) and the two other responses lasting 23 and 33 months. The two (2) remaining Merkel cell carcinoma patients were not evaluable for response from IO rechallenge due to radiation administered to the only measurable disease site(s), but both remain progression free at 11+ and 13+ months with previously progressive disease.
Of the twelve (12) patients with advanced Merkel cell carcinoma who completed treatment and protocol-directed 28-day safety evaluation follow-up period, seven patients exhibited primary resistance to first line treatment with a checkpoint inhibitor who did not receive subsequent therapies prior to receiving IFx-2.0. Five of seven patients received single agent anti-PD(L)-1 as initial therapy while two of seven patients received multiple CPIs as initial therapy including anti-PD-1, followed by anti-PD-1/anti-CTLA-4 therapy. All 7 patients exhibited primary resistance to checkpoint inhibitor therapy progressing on average 3.3 months while receiving CPI therapy. These 7 patients are graphically presented below:
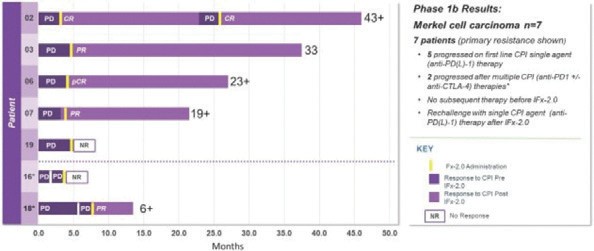
This data demonstrating the potential for IFx-2.0 to overcome primary resistance to anti-PD(L)-1 therapy and formed the clinical rationale for examining IFx-2.0 as adjunctive therapy with Keytruda® (anti-PD-1) in first line therapy among checkpoint
17
inhibitor naïve patients with advanced or metastatic Merkel cell carcinoma. Unlike the phase 1b where IFx-2.0 was administered after patients progressing on anti-PD(L)-1 therapy, we believe IFx-2.0 could potentially provide a higher response rate to Keytruda® when administered prior to patients progressing failing Keytruda®.
The remaining seven (7) patients received multiple checkpoint inhibitor therapy including anti- CTLA-4/anti-PD-1 therapy and/or investigational agent(s) and or chemotherapy as 2nd or 3rd line therapy prior to treatment with IFx-2.0. This patient population is not representative of patients to be enrolled in the phase 3 trial.
Importantly, IFx-2.0 is not an intratumoral therapy like oncolytic viral therapies whose anti-tumor activity is limited to accessible, injected lesions in limited stages of cancer. In contrast, IFx-2.0’s mechanism of action is to prime and activate an innate immune response in injected lesions leading to a systemic anti-tumor response. The Company chose to examine IFx-2.0 in cutaneous malignancies because human skin has a high density of DCs which are very efficient in presenting foreign antigens to immune cells. Local injection of IFx-2.0 into cutaneous lesion(s) has resulted in immune cell infiltration, and in the context of MHCI and MHCII, tumor neoepitope presentation to naïve B and T cells followed by activation of tumor specific B and T cells. The immune response has not been localized to just injected lesions but rather systemic as demonstrated by production of Emm55 (pDNA encoded bacterial protein expressed on the surface of the tumor cell) and tumor specific IgM and IgG antibodies in the plasma of patients post IFx-2.0 administration.
Patients Merkel cell carcinoma-03 and Merkel cell carcinoma-05 below demonstrate the abscopal effect of adjunctive IFx-2.0 therapy, These patients exhibited primary resistance to checkpoint inhibitor therapy, and subsequently achieved durable anti- tumor responses following IFx-2.0 and rechallenge with checkpoint inhibitor therapy.
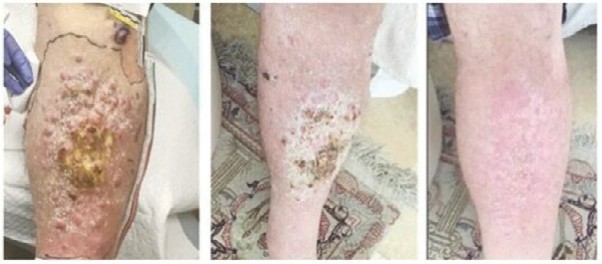
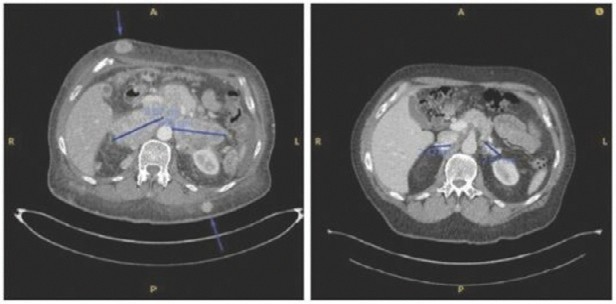
Case study (MCC-005)
Patient was treated for multifocal in-transit recurrence of Merkel cell carcinoma in left leg with avelumab x 6 doses (12 weeks) with continued rapid clinical progression as well as development of liver metastatic disease on this therapy. Subsequently the patient was enrolled on IFx-2.0 protocol and received 3 weekly injections of IFx-2.0 without complication but continued clinical progression (additional in-transit sites). Disease status at time of last injection shown on the left. Following completion of IFx-2.0 protocol therapy, subject was rechallenged with pembrolizumab, a checkpoint inhibitor, and experienced an obvious clinical response initially apparent approximately 3-4 weeks into therapy. Clinical response at 3 months (middle photo below) and 6 months (right photo below) are shown in the photos below. Concordant (near-complete) radiographic response of liver metastases has also been observed and response has been maintained to date (19 months)
18
Case study (MCC-002)
Subject was treated with adjuvant pembrolizumab for stage II Merkel cell carcinoma on the STAMP trial but developed (nodal) progression after receiving 6 doses. Subject underwent salvage surgery/XRT but developed widespread metastatic disease ~3 months later (nodal, dermal, and intramuscular sites of disease). Subject was then enrolled on IFx-2.0 protocol and received 2 weekly injections to 3 nodal/dermal metastatic sites but experienced continued rapid progression (both injected and non-injected sites) including bulky diffuse adenopathy and numerous widespread subcutaneous/dermal nodules. Representative imaging from the time of completion of protocol therapy is shown on left in photo below including several subcutaneous sites (as noted by the arrows) and bulky retroperitoneal (“RP”) conglomerate lymph node (“LN”) metastases. Post-protocol, subject was started on checkpoint inhibitor rechallenge with avelumab and experienced deep partial response that has been maintained to date (33 months). Representative images from post-checkpoint rechallenge restaging shown below on right (complete remission of subcutaneous nodules, partial response in retroperitoneal sites).
IFx-2.0 Planned Basket Trial
We are planning a Phase 1b/2a trial referred to as a “basket” trial, which is a type of clinical trial that tests a new product candidate in patients who have different types of cancer but a common biologic reason for resistance to checkpoint inhibitors. The Phase 1b stage of the trial will examine the feasibility and safety of Keytruda® and adjunctive IFx-2.0 where IFx-2.0 is administered via interventional radiology into lesions in the liver, retroperitoneal or lungs of patients who have advanced and metastatic Merkel cell carcinoma who are checkpoint inhibitor naïve. Following the Phase 1b component, the planned Phase 2a stage will extend enrollment to patients with any cancer type (so-called “histology agnostic”) who exhibit a high incidence of primary resistance to checkpoint inhibitors in cancers such as advanced triple negative breast cancer or ovarian cancer. Since the biology of primary resistance to checkpoint inhibitors is similar across tumor types. We believe that IFx-2.0’s mechanism of action should be applicable in overcoming primary resistance to checkpoint inhibitors irrespective of tumor type. We currently anticipate initiating this study in the second half of 2025. If successful, this trial could have the ability to expand the utility of IFx-2.0 beyond advanced Merkel cell carcinoma.
Phase 1 Trial in Advanced, (Stage IIIC-IV) Cutaneous Melanoma
We also conducted a Phase 1 trial at the Moffitt Cancer Center in seven (7) patients with advanced (Stage IIIc/IV) cutaneous melanoma, six (6) of whom were eligible for evaluation post-IFx-2.0 therapy. The primary objective of the trial was to determine the safety and tolerability of IFx-2.0 when administered intratumorally with up to three lesions injected at a single time point. Safety was evaluated for 28 days following IFx-2.0 administration. Secondary objectives included tumor shrinkage, transcriptomic, proteomic, humoral, and cellular immune response pre and post IFx-2.0 administration. IFx-2.0 was well tolerated. Mild pain and swelling among injected lesions were most common reported side effect < Grade 2 in severity. Four (4) of the six (6) patients exhibited primary resistance to, and failed checkpoint inhibitor trials prior to IFx-2.0. Following IFx-2.0 administration three (3) of four (4) patients subsequently responded to rechallenge with checkpoint inhibitor(s). One patient achieved stable disease (“SD”) and 2 experienced a partial response (“PR”). As of the last follow up responses are ongoing at 1337, 608, 313 days. Two (2) patients (SD and PR) underwent surgical resections following checkpoint inhibitor therapy. Immunologic profiling data (pre-and post-IFx-2.0) demonstrated
19
a robust systemic immune response with (i) activation of tumor specific B cells with tumor specific IgM/IgG antibody production recognizing hundreds of previously unrecognized melanoma tumor neoepitopes and (ii) gene signature, consistent with innate response in injected lesions, a gene signature consistent with adaptive response in un-injected lesions as well as increased expression (up to 11 fold) of genes known to be predictive of response to checkpoint inhibitors following IFx-2.0 therapy but prior to checkpoint inhibitor rechallenge.
Planned IND-Enabling Studies for IFx-3.0 Next Generation mRNA Innate Immune Agonist
We are also developing a second innate immune agonist candidate that incorporates our codon optimized mRNA into a lipid nanoparticle coupled to an antibody f targeting the CD22 receptor. CD22 is overexpressed on a variety of B cell cancers including aggressive lymphomas like diffuse large B cell lymphoma or DLBCL. Unlike IFx-2.0, which utilizes a proprietary pDNA for intratumoral administration, we are designing IFx-3.0 for intravenous (or autologous whole cell) administration. This is intended to allow extension of our innate immune agonist candidates to tumors not accessible by injection, like blood-related cancers, and could result in eliciting a more potent immune response without the need for checkpoint inhibitors. We plan to test various constructs in vitrobefore advancing to humanized immune competent murine models of aggressive lymphoma planned for 2026.
Market Opportunity
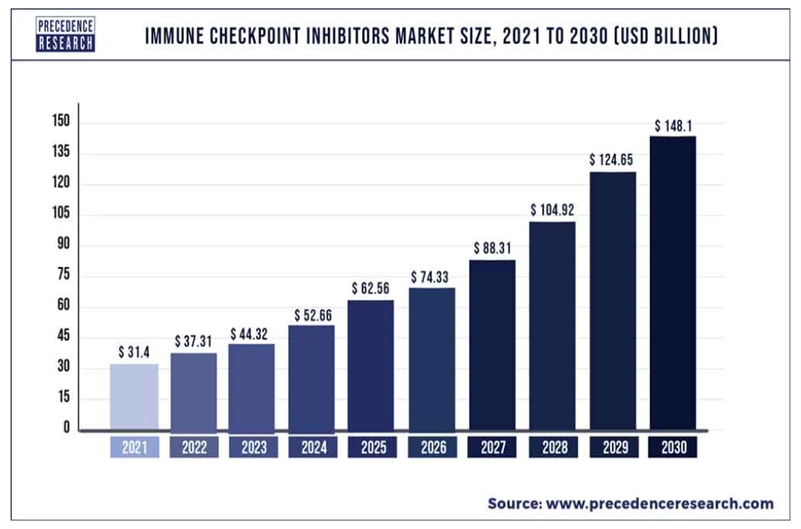
Checkpoint inhibitors dominate oncology sales and represent the most successful oncology drug commercial launches in oncology drug development. Since their commercial launch in 2014, sales of checkpoint inhibitors have grown at an impressive compounded annual growth rate with $29.9 billion in sales in 2020 reaching $37 billion in 2022, according to Precedence Research. By 2030 the market is expected to grow to over $148 billion in world wide sales, according to Precedence Research. We believe that our technology platforms have the potential to address both primary and acquired resistance, the two major limitations to checkpoint inhibitor and cellular therapies and as such represents a large market opportunity. While upward of 15% to 60% of patients will respond to first time treatment with checkpoint inhibitors, 40% to 85% will not. It is this population of patients with primary resistance to checkpoint inhibitors that we believe represents the initial market opportunity for IFx-2.0. The biologic basis for primary resistance to checkpoint inhibitors is similar across various tumor types, predominately the lack of tumor infiltration with activated tumor specific T cells. We believe that an agent that can overcome primary resistance to checkpoint inhibitors in one tumor type should overcome resistance in others, if not all, tumor types that exhibit primary resistance to them. Our initial strategy is to demonstrate the ability of IFx-2.0 to overcome primary resistance in the 50% of patients with advanced Merkel cell carcinoma receiving front line therapy with Keytruda® (pembrolizumab), the current standard of care, allowing more patients to achieve an anti-tumor response than with Keytruda® alone.
20
According to DelveInsight, it is estimated by 2027 there will be approximately 4,245 patients in the US and 7,049 patients in the 7 major market European countries, including the UK, growing to a total of 15, 262 patients by 2034 in these geographic territories. The standard of care for patients with the advanced or metastatic Merkel cell carcinoma is therapy with a checkpoint inhibitor like Keytruda® (pembrolizumab). If the results of our above-described “basket” trial are successful, the results from that clinical trial could allow IFx-2.0 to be used in a variety of tumor types other than Merkel cell carcinoma that exhibit primary resistance to checkpoint inhibitors, which could expand the market application of IFx-2.0 significantly.
Among patients who initially respond to treatment with checkpoint inhibitors, almost all patients will ultimately develop acquired resistance where checkpoint inhibitors no longer work and the tumor recurs and/or progresses. While the cause of acquired resistance is multifactorial, a major contributor is tumor associated MDSC-induced immunosuppression of the tumor microenvironment leading to T cell exhaustion and failure of checkpoint inhibitors or cellular therapies. Our initial strategy is to investigate our MDSC-targeted bi-functional ADCs in tumor types that initially responded to and subsequently progressed on or following checkpoint inhibitor therapy. If successful in overcoming acquired resistance to checkpoint inhibitors while potentially limiting their toxicity to non-tumor tissue, such an application would be expected to also represent a significant market opportunity.
Our Manufacturing Strategy
We are working with a number of contract development and manufacturing contract organizations (CDMOs) to produce product candidate components, clinical trial material as well as cGMP drug substance and drug product and necessary validated analytical tests required for registration trials and commercial material. We may enter into development collaborations with large pharmaceutical or biotech companies where we would look to our development partner to assume responsibility for product manufacturing and supply.
We utilize CDMOs to make the emm55-pDNA, drug substance, and drug product. The emm55-pDNA utilizes a cationic polymer as a transfectant agent excipient and is mixed with dextrose at the site of administration. As is common practice for drug products requiring mixing at site of administration, the FDA requires standard mixing studies to be published in the pharmacy manual to guide correct process for constitution of the drug product prior to administration. In addition, the FDA requires potency assay(s) and stability assays among other standard processes to allow specifications from batch to batch to meet pre-specified agreed to assay parameters allowing product release for clinical trials. We, through our third party CDMOs, are in the process of completing development, qualification and validation of all such assays necessary for the production and release of drug product which meets cGMP requirements for use in our Phase 3 registration directed trial.
Intellectual Property
Intellectual property is of vital importance in our field and in biotechnology generally. We seek to protect and enhance proprietary technology, inventions, and improvements that are commercially important to the development of our business by seeking, maintaining, and defending patent rights, whether developed internally or licensed from third parties. We also seek to rely on regulatory protection afforded through inclusion in expedited development and review, data exclusivity, market exclusivity and patent term extensions where available. We have sought patent protection in the United States and internationally related to our IFx-Hu2.0 platform technology as well as our IFx-Hu3.0 technology, and we license from third parties the patents and patent applications relating to our tumor microenviroment modulators technology.
We expect to file additional patent applications in support of current and new clinical candidates, as well as new platform and core technologies. Our commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection of our current and future product candidates and the methods used to develop and manufacture them, as well as successfully defending any such patents against third-party challenges and operating without infringing on the proprietary rights of others. Our ability to stop third parties from making, using, selling, offering to sell or importing our product candidates will depend on the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities.
The terms of individual patents depend upon the statutory term of the patents in the countries in which they are issued. In most countries in which we file, including the United States, the patent term is 20 years from the earliest filing of a non-provisional patent application. In the United States, a patent term may be lengthened by patent term adjustment (“PTA”), which compensates a patentee for administrative delays by the USPTO in examining and granting a patent. Conversely, a patent term may be shortened if a patent is terminally disclaimed over an earlier filed patent. In the United States, the term of a patent that covers an FDA-approved drug may also be eligible for extension, which permits patent term restoration to account for the patent term lost during the FDA regulatory review process. The Hatch-Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the subject drug candidate is under regulatory review. Patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent applicable to an approved drug may be extended and only those claims covering the approved drug, a method for using it, or a method
21
for manufacturing it may be extended. Similar provisions to extend the term of a patent that covers an approved drug are available in Europe and other foreign jurisdictions. In the future, if and when our products receive FDA approval, we expect to apply for patent term extensions on patents covering those products. We plan to seek patent term extensions to any issued patents we may obtain in any jurisdiction where such patent term extensions are available, however there is no guarantee that the applicable authorities, including the FDA in the United States, will agree with our assessment that such extensions should be granted, and if granted, the length of such extensions.
In some instances, we have submitted and expect to submit patent applications directly to the USPTO as provisional patent applications. Corresponding non-provisional patent applications must be filed not later than 12 months after the provisional application filing date. While we intend to timely file non-provisional patent applications relating to our provisional patent applications, we cannot predict whether any such patent applications will result in the issuance of patents that provide us with any competitive advantage.
We expect to file U.S. non-provisional applications and Patent Cooperation Treaty, or PCT, applications that claim the benefit of the priority date of earlier filed provisional applications, when applicable. The PCT system allows a single application to be filed within 12 months of the original priority date of the patent application and to designate all of the PCT member states in which national patent applications can later be pursued based on the international patent application filed under the PCT. A designated authority performs an initial search and issues a non-binding opinion as to the patentability of the subject matter. The opinion may be used to evaluate the chances of success of national phase applications in various jurisdictions, thereby informing the development of a global filing strategy.
Although a PCT application does not itself issue as a patent, it allows the applicant to conveniently file applications in any of the member states through national-phase applications. At the end of a period of 30-31 months from the earliest priority date of the patent application (varies by jurisdiction), individual applications can be filed in any of the PCT member states/regions. Use of the PCT system is more cost-effective than direct foreign filings and permits applicants greater flexibility with respect to budgeting and the selection of foreign jurisdictions.
For all patent applications, we determine claiming strategy on a case-by-case basis. Advice of counsel and our business model and needs are always considered. We seek to file patents containing claims for protection of all useful applications of our proprietary technologies and any products, as well as all new applications and/or uses we discover for existing technologies and products, assuming these are strategically valuable. We continuously reassess the number and type of patent applications, as well as the pending and issued patent claims to pursue maximum coverage and value for our processes, and compositions, given existing patent office rules and regulations. Further, claims may be modified during patent prosecution to meet our intellectual property and business needs.
We recognize that the ability to obtain patent protection and the degree of such protection depends on a number of factors, including the extent of the prior art, the novelty and non-obviousness of the invention, and the ability to satisfy the enablement requirement of the patent laws. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted or further altered even after patent issuance. Consequently, we may not obtain or maintain adequate patent protection for any of our future product candidates or for our technology platform. We cannot predict whether the patent applications we are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient proprietary protection from competitors. Any patents that we hold may be challenged, circumvented or invalidated by third parties.
The patent positions of biotechnology companies are generally uncertain and involve complex legal, scientific and factual questions. Our commercial success will also depend in part on not infringing upon the proprietary rights of third parties. Third-party patents could require us to alter our development or commercial strategies, or our products or processes, obtain licenses or cease certain activities. Our breach of any license agreements or our failure to obtain a license to proprietary rights required to develop or commercialize our future products may have a material adverse impact on us.
If third parties prepare and file patent applications in the United States that also claim technology to which we have rights, we may have to participate in interference or derivation proceedings in the USPTO to determine priority of invention. For more information, see “Risk Factors – Risks Relating to Our Intellectual Property.”
When available to expand market exclusivity, our strategy is to obtain, or license additional intellectual property related to current or contemplated development platforms, core elements of technology and/ or clinical candidates.
22
Company-owned Intellectual Property
As of December 31, 2024, we had 33 issued patents over 13 jurisdictions, and 9 pending applications (2 U.S. utility patent applications and 7 foreign patent applications). Most of such patents and patent applications relate to our IFx technology platform. The following is a summary of our issued patents and pending patent applications as of December 31, 2024 by patent family.
Patent Family |
|
Description |
|
Application/Publication/ Patent Number |
|
Filing Date |
|
Issue Date/Status |
|
Earliest Expected Expiration Date |
|
Type of Parent Protection |
|
|
|
|
|
|
|
|
|
|
|
|
|
DNA Vector and Transformed Tumor Cell Vaccines |
|
Whole cell and DNA cancer vaccines |
|
PCT/US2015/018688 (WO 2015/134577) |
|
03/04/2015 |
|
Nationalized in CH, DE, DK, EP, FR, GB, HK, IE, NL, NO, SE, US |
|
03/04/2035 |
|
Use Composition Composition |
|
|
US 9,555,088 US 9,839,680 US 10,391,158 US 10,751,400 |
|
07/07/2016 01/30/2017 12/11/2017 08/26/2019 |
|
Issued 01/31/2017 Issued 12/12/2017 Issued 08/27/2019 Issued 08/25/2020 |
|
03/4/2035 03/4/2035 03/4/2035 03/4/2035 |
|
Use |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
Cancer Vaccine Comprising mRNA Encoding a M-Like- Protein |
|
Next generation cancer vaccine using mRNA encoding a bacterial antigen to prime anti- cancer immune responses |
|
PCT/US2016/033235 (WO 2016/187407)
US 9,636,388 US 10,682,401 US 18/060,605 |
|
05/19/2016
07/28/2016 05/01/2017 12/01/2022 |
|
Nationalized in AU, CA, CH, CN, DE, DK, EP, FR, GB, HK, IE, JP, NL, NO, SE, US
Issued 05/02/2017 Issued 06/16/2020 pending |
|
05/19/2036 05/19/2036 05/19/2036 |
|
Use Composition Composition/use |
|
|
|
|
|
|
|
|
|
|
|
|
|
Modified mRNA for Multicell Transformation |
|
Next generation cancer vaccine using mRNA encoding a bacterial antigen to prime anti- cancer immune responses |
|
PCT/US2021/031204 (WO 2021/226413) |
|
05/7/2021 |
|
Nationalized in CN, JP, CA, IN, AU, EP, KR To be filed in HK |
|
05/7/2041 |
|
|
Exosome Delivery of Cancer Therapeutics |
|
Production and use of exosome preparations to systemically deliver pDNA and/or |
|
US 18/055,724
|
|
11/15/2022 |
|
Published/pending
|
|
|
|
Composition/use |
Licensed Intellectual Property Rights Relating to Delta Receptor Technology
We license the intellectual property rights relating to our tumor microenviroment modulator technology platform under exclusive license agreements with H. Lee Moffitt Cancer Center and Research Institute (“Moffitt Cancer Center”) and the West Virginia University Research Corporation (“WVURC”). In particular, we are a party to a March 2019 Exclusive License Agreement with Moffitt Cancer Center under which, as amended, we license patent rights co-owned by Moffitt and University of South Florida relating to ADCs for immunotherapy and Delta receptor targeted agents for molecular imaging and immunotherapy of lung cancer. We are a party to a second Exclusive License Agreement entered into in April 2021 under which, as amended, we license Moffitt’s interest in certain patent rights relating to the applicability of our Delta receptor technology to the tumor microenvironment (these patent rights are co-owned by Moffitt and us). We are a party to a September 2022 Restated and Amended Exclusive License Agreement with WVURC pursuant to which we license from WVURC certain patent rights (including WVURC’s rights under one patent that is jointly owned by WVURC and our company) relating to Delta receptor targeted agents for molecular imaging and cancer immunotherapy.
These license agreements were originally entered into with Moffitt and WVURC by TuHURA Biopharma, which assigned its interest under the agreements to us as a part of the acquisition of certain TuHURA Biopharma assets in January 2023. The following are summaries of the material terms of these license agreements:
2019 License Agreement with Moffitt Cancer Center
In March 2019, TuHURA Biopharma, as predecessor in interest to the Company, entered into an Exclusive License Agreement with Moffitt Cancer Center, which agreement was amended in September 2019, April 2021 and August 2022 (as amended, the “2019 Moffitt Agreement”), for the worldwide, exclusive license of patents for the development, commercialization and marketing of products derived from Moffitt’s rights to patents entitled “Conjugates for Immunotherapy” and “A Delta-Opioid Receptor Targeted
23
Agent For Molecular Imaging And Immunotherapy Of Lung Cancer” (the “2019 Moffitt Licensed Patents”). The exclusive nature of the granted licenses are subject to customary reservations by Moffitt for non-commercial research, development, and academic purposes. The licenses granted by Moffitt are sublicensable by us to affiliates and third parties, subject to certain requirements, including providing Moffitt with a copy of each executed sublicense agreement and ensuring that the sublicensee complies with the terms of the 2019 Moffitt Agreement.
Pursuant to the terms of the 2019 Moffitt Agreement, in partial consideration of Moffitt’s grant of the rights and licenses, TuHURA Biopharma paid to Moffitt one-time, non-refundable license issue fees of $100,000 and $30,000. Additionally, TuHURA Biopharma issued shares of its common stock to Moffitt as additional consideration, which were exchanged for 146,397 shares of our common stock as a part of the TuHURA Biopharma asset acquisition. We are obligated to pay Moffitt an annual license maintenance fee not in excess of $50,000 per year until annual minimum royalty payments commence following commercial sales of licensed products.
Also under the 2019 Moffit Agreement, we are required to make the following additional payments:
The term of the 2019 Moffitt Agreement will be until the later of (i) the date on which the last of the licensed patents expire, or (ii) twenty (20) years after the date of the 2019 Moffitt Agreement. We may unilaterally terminate the 2019 Moffitt Agreement at any time on six (6) months’ notice to Moffitt, provided that all payments due by us at that time have been made through the effective date of termination. Additionally, we may terminate the agreement with written notice to Moffitt in the event Moffitt commits a material breach and such breach is not cured within sixty (60) days following Moffitt’s receipt of such notice. Moffitt has the right to terminate, or convert all exclusive licenses to nonexclusive licenses in the event we: (x) fail to make payments due under the agreement within thirty (30) days following notice from Moffitt; (y) commit a material breach that is not cured, or capable of being cured, within sixty (60) days after receipt of notice from Moffitt; (z) or challenge the validity of any of the 2019 Moffitt Licensed Patents before a court or other administrative agency in any jurisdiction. Upon any termination prior to the expiration of the agreement for any reason, all licenses and rights granted pursuant to the agreement will automatically terminate. At the request of Moffitt, we are obligated to provide all materials, clinical results, regulatory submissions, registrations and any other related filings for the 2019 Moffitt Licensed Patents, and all data used to support the same, to Moffitt.
2021 License Agreement with Moffitt Cancer Center
In April 2021, TuHURA Biopharma, as predecessor in interest to us, entered into an Exclusive License Agreement with Moffitt, which agreement was amended in August 2022 (collectively, the “2021 Moffitt Agreement”), for the worldwide, exclusive, license to Moffitt’s rights under a jointly-owned patent entitled “Delta Opioid Receptor Antagonist Reprogram Immunosuppressive Microenvironment to Boost Immunotherapy” (the “2021 Moffitt Licensed Patent”) for the development, commercialization and marketing of products from covered claims of the 2021 Moffitt Licensed Patent. The exclusive nature of the licenses granted are subject to customary reservations by Moffitt for non-commercial research, development, and academic purposes. The licenses granted by Moffitt are sublicensable by the Company to affiliates and third parties, subject to certain requirements, including providing Moffitt with a copy of each executed sublicense agreement, and ensuring that the sublicensee comply with the terms of the 2021 Moffitt Agreement.
Pursuant to the terms of the 2021 Moffitt Agreement, in partial consideration of Moffitt’s grant of the rights and licenses, TuHURA Biopharma paid to Moffitt a one-time, non-refundable license issue fee of $12,500. Additionally, TuHURA Biopharma issued shares of its common stock to Moffitt as additional consideration, which were exchanged for 195,465 shares of our common stock as a part of the TuHURA Biopharma asset acquisition. We are obligated to pay Moffitt an annual license maintenance fee not in excess of $25,000 per year until annual minimum royalty payments commence following commercial sales of licensed products.
We are also required to make the following additional payments:
24
The term of the 2021 Moffitt Agreement will be until the later of (i) the date on which the last of the patents expire, or (ii) twenty (20) years after the date of the 2021 Moffitt Agreement. We may unilaterally terminate the 2021 Moffitt Agreement at any time on six (6) months’ notice to Moffitt, provided that all payments due by us at that time have been made through the effective date of termination. Additionally, we may terminate the agreement with written notice to Moffitt in the event Moffitt commits a material breach and such breach is not cured within sixty (60) days following Moffitt’s receipt of such notice. Moffitt has the right to terminate, or convert all exclusive licenses to nonexclusive licenses in the event we: (x) fail to make payments due under the agreement within thirty (30) days following notice from Moffitt; (y) commit a material breach that is not cured, or capable of being cured, within sixty (60) days after receipt of notice from Moffitt; (z) or challenge the validity of any of the 2021 Moffitt Licensed Patent before a court or other administrative agency in any jurisdiction. Upon any termination prior to the expiration of the agreement for any reason, all licenses and rights granted pursuant to the agreement will automatically terminate. At the request of Moffitt, we are obligated to provide all materials, clinical results, regulatory submissions, registrations and any other related filings for the 2021 Moffitt Licensed Patent, and all data used to support the same, to Moffitt.
License Agreement with West Virginia University Research Corporation
In January 2023 but with an effective date of September 2022, TuHURA Biopharma, as predecessor in interest of us, entered into a Restated and Amended Exclusive License Agreement with WVURC (the “WVU Agreement”), which terminated and replaced the prior agreement between WVURC and TuHURA Biopharma. The WVU Agreement provides for the exclusive commercialization rights relating to Delta receptor targeted agents for WVURC patent rights relating to molecular imaging and cancer immunotherapies (the “WVU Patents”). Under the WVU Agreement, among other rights, WVURC granted us a worldwide, exclusive right, with limited sublicense rights, to develop and commercialize the WVU Patents in accordance with the milestone schedule therein.
As partial consideration for the rights granted under the WVU Agreement, TuHURA Biopharma previously paid a non-refundable, upfront fee of $50,000. Under the terms of the WVU Agreement, we are required to pay WVURC a tiered running royalty in the low-to-mid single digit percentages based on levels of net sales of licensed products, including the net sales of sublicensees, with customary anti-stacking provisions. We are also required to pay annual fees of $30,000 or less and is required to fund all patent prosecution and maintenance costs and fees for the licensed patents.
The term of the WVU Agreement will expire on the later of: (i) the expiration of the date of the last to expire of the WVU Patents or (ii) twenty (20) years from the first commercial sale of a licensed product derived from the WVU Patents, unless earlier terminated pursuant to its terms. We may unilaterally terminate the WVU Agreement upon written notice to WVURC at any time on six (6) months’ notice to WVURC, provided that all payments due by us at that time have been made through the effective date of termination. Additionally, we may terminate the agreement with written notice to WVURC in the event WVURC commits a material breach and such breach is not cured within sixty (60) days following WVURC’s receipt of such notice. WVURC has the right to terminate, or convert all exclusive licenses to nonexclusive licenses in the event we fail to make payments due under the agreement within thirty (30) days following notice from WVURC; commit a material breach that is not cured, or capable of being cured, within ninety (90) days after receipt of notice from WVURC; or challenge the validity of any of the WVU Patents before a court or other administrative agency in any jurisdiction. Upon any termination prior to the expiration of the WVU Agreement for any reason, all licenses and rights granted pursuant to the agreement will automatically terminate.
25
The following is a summary of the patent rights licensed from Moffitt Cancer Center and WVURC:
Patent Family |
|
Description |
|
Application/Publication/ Patent Number |
|
Filing Date |
|
Issue Date/Status |
|
Earliest Expected Expiration Date |
|
Type of Patent Protection |
|
|
|
|
|
|
|
|
|
|
|
|
|
DNA Vector and Transformed Tumor Cell Vaccines |
|
Whole cell and DNA cancer vaccines |
|
PCT/US2015/018688 (WO 2015/134577) |
03/04/2015 |
|
Nationalized in CH, DE, DK, EP, FR, GB, HK, IE, NL, NO, SE, US |
|
03/04/2035 |
|
Use Composition Composition/use |
|
|
|
|
|
|
|
|||||||
|
|
|
|
US 9,555,088 US 9,839,680 US 10,391,158 US 10,751,400
|
07/07/2016 01/30/2017 12/11/2017 08/26/2019 |
|
Issued 01/31/2017 Issued 12/12/2017 Issued 08/27/2019 Issued 08/25/2020 |
|
3/4/2035 3/4/2035 3/4/2035 3/4/2035 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cancer Vaccine Comprising mRNA Encoding a M-Like-Protein |
|
Next generation cancer vaccine using mRNA encoding a bacterial antigen to prime anti- cancer immune responses |
|
PCT/US2016/033235 (WO 2016/187407)
US 9,636,388 US 10,682,401 US 18/060,605
|
05/19/2016
07/28/2016 05/01/2017 12/01/2022
|
Nationalized in AU, CA, CH,CN, DE, DK, EP, FR, GB, HK, IE, JP, NL, NO, SE, US
Issued 05/02/2017 Issued 06/16/2020 pending
|
|
5/19/2036 5/19/2036 5/19/2036
|
|
Use Composition Composition/use |
||
|
|
|||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
Modified mRNA for Multicell Transformation |
|
Next generation cancer vaccine using mRNA encoding a bacterial antigen to prime anti- cancer immune responses
|
PCT/US2021/031204 (WO 2021/226413) |
5/7/2021 |
Nationalized in CN, JP, CA, IN, AU, EP, KR To be filed in HK |
5/7/2041 |
|
|||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
Exosome Delivery of Cancer Therapeutics |
|
Production and use of exosome preparations to systemically deliver pDNA and/or mRNA tumors |
US 18/055,724 (US 2023-0183690)
|
11/15/2022 |
Published/ pending |
|
Composition/use |
|||||
|
||||||||||||
|
||||||||||||
|
Employees and Human Capital Resources
As of December 31, 2024, we had 19 full-time employees and no part-time employees. Of these employees, 15 were engaged in research and development activities. The majority of our employees are based in Tampa, Florida. None of our employees are represented by labor unions or covered by collective bargaining agreements. We consider our relationship with our employees to be good.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and new employees, advisors and consultants. The principal purposes of our equity and cash incentive plans are to attract, retain and reward personnel through the granting of stock-based and cash-based compensation awards, in order to increase stockholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives.
Government Regulation and Product Approval
Therapeutic products are subject to rigorous regulation by the FDA and other governmental agency regulations in the United States and in foreign countries. Noncompliance with applicable requirements can result in import detentions, fines, civil monetary penalties, injunctions, suspensions or losses of regulatory approvals or licenses, recall or seizure of products, operating restrictions, denial of export applications, governmental prohibitions on entering into supply contracts, and criminal penalties and prosecution. Failure to obtain regulatory approvals or the restriction, suspension or revocation of regulatory approvals or licenses, as well as any
26
other failure to comply with regulatory requirements, would have a material adverse effect on our business, financial condition and results of operations. In connection with seeking therapeutic approval, we will have to comply with the many regulations and requirements associated with the conduct of preclinical and clinical trials, the FDA application process, the terms of any pre-certification protocols and agreements, FDA manufacturing requirements for investigational products, and testing. Upon approval of a Biologics License Application, or BLA, and similar approvals in other jurisdictions, there will be additional regulations that must be complied with, including regulations relating to the packaging, distribution, marking, marketing and claims of our potential products. These later regulations are not only found in federal regulation but many states and, of course, foreign countries.
The U.S. FDA Process
The FDA regulates the clinical testing and design of therapeutics to ensure that medical products distributed in the United States are safe and effective for their intended uses. The application process for a new therapeutic is highly regulated.
As a biopharmaceutical company that operates in the United States, we are subject to extensive regulation by relevant authorities, including the FDA. Our potential products will be regulated as biologics. With this classification, commercial production of its potential products will need to occur in registered and licensed facilities in compliance with current good manufacturing practices (cGMP) established by the FDA for biologics. The FDA categorizes human cell- or tissue-based products as either minimally manipulated or more than minimally manipulated, and has determined that more than minimally manipulated products require clinical trials to demonstrate product safety and efficacy and the submission of a BLA for marketing authorization.
Government authorities in the United States (at the federal, state and local levels) and in other countries extensively regulate, among other things, the research, development, testing, manufacturing, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of biopharmaceutical products such as those we are developing. Our candidates must be approved by the FDA before they may be legally marketed in the United States and by the appropriate foreign regulatory agency before they may be legally marketed in a foreign country. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the United States, although there can be important differences. Additionally, some significant aspects of regulation in Europe are addressed in a centralized way, but country-specific regulation remains essential in many respects. The process for obtaining regulatory marketing approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources.
U.S. Product Development Process
In the United States, the FDA regulates pharmaceutical and biological products under the Federal Food, Drug, and Cosmetic Act, or FDCA, the Public Health Service Act, or PHSA, and their respective implementing regulations. Products are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. FDA sanctions could include, among other actions, refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning letters, product recalls or withdrawals from the market, product seizures, total or partial suspension of production or distribution injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us. The FDA has limited experience with commercial development of T cell therapies for cancer, including direct- injectable technologies such as AIM INJ. The process required by the FDA before a biological product may be marketed in the United States generally involves the following:
27
Preclinical studies
Before testing any biological product candidate, including our drug candidates, in humans, the drug candidate enters the preclinical testing stage. Preclinical tests, also referred to as nonclinical studies, include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies to assess the potential safety and activity of the drug candidate. The conduct of the preclinical tests must comply with federal regulations and requirements, including GLP. The clinical trial sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND. Some preclinical testing may continue even after the IND is submitted. An IND is a request for authorization from the FDA to administer an investigational product to humans, and must become effective before human clinical trials may begin.
Human clinical trials in support of a BLA
Clinical trials involve the administration of the biological product candidate to human research subjects under the supervision of qualified investigators, generally licensed physicians not employed by or under the trial sponsor’s control. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection, inclusion and exclusion criteria, and the parameters to be used to monitor subject safety, including stopping rules that assure a clinical trial will be stopped if certain adverse events should occur. Each protocol and any amendments to the protocol must be submitted to the FDA as part of the IND. An IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions regarding the proposed clinical trials and places the trial on a clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. The FDA may also impose clinical holds on a biological product candidate at any time before or during a clinical trial due to safety concerns or non-compliance. If the FDA imposes a clinical hold, the trial may not recommence without FDA authorization and then only under terms authorized by the FDA. Accordingly, we cannot be sure that submission of an IND will result in the FDA allowing clinical trials to begin or that, once begun, issues will not arise that suspend or terminate such trials.
Clinical trials must be conducted and monitored in accordance with the FDA’s regulations comprising the GCP requirements, including the requirement that all research participants provide informed consent. Further, each clinical trial must be reviewed and approved by an independent institutional review board, or IRB, at or servicing each institution at which the clinical trial will be conducted. An IRB is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the form and content of the informed consent form that must be signed by each clinical trial subject or the participant’s legal representative and must monitor the clinical trial until completed. For certain clinical trials involving biologics, they also must be reviewed by an institutional biosafety committee, or IBC, a local institutional committee that reviews and oversees basic and clinical research conducted at that institution. The IBC assesses the safety of the research and identifies any potential risk to public health or the environment.
28
Information about certain clinical trials, including details of the protocol and eventually study results, also must be submitted within specific timeframes to the National Institutes of Health, or NIH, for public dissemination on the ClinicalTrials.gov data registry. Information related to the investigational product, patient population, phase of investigation, study sites and investigators and other aspects of the clinical trial is made public as part of the registration of the clinical trial. Sponsors are also obligated to disclose the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed in some cases for up to two years after the date of completion of the trial.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
Post-approval clinical trials, sometimes referred to as phase 4 clinical trials, may be conducted after initial marketing approval. These clinical trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication, particularly for long-term safety follow-up. In certain instances, the FDA may mandate the performance of phase 4 clinical trials as a condition of approval of a BLA.
Progress reports detailing the results of the clinical trial must be submitted at least annually to the FDA and more frequently if serious adverse events, or SAEs, occur. The FDA or the trial sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research participants are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the clinical protocol, GCP, or other IRB requirements, or if the investigational product has been associated with unexpected serious harm to patients. Additionally, some trials are overseen by an independent group of qualified experts organized by the trial sponsor known as the data safety monitoring board or committee. This group provides authorization for whether a trial may move forward at designated checkpoints.
During the development of a new drug or biological product, sponsors have the opportunity to meet with the FDA at certain points, including prior to submission of an IND, at the end of phase 2, and before submission of a BLA. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide guidance, and for the sponsor and the FDA to reach agreement on the next phase of development. Sponsors typically use the end of phase 2 meeting to discuss their phase 2 clinical results with the agency and to present their plans for the pivotal phase 3 studies that they believe will support approval of the new drug or biological product.
Human immunotherapy products are a new category of therapeutics. Because this is a relatively new and expanding area of novel therapeutic interventions, there can be no assurance as to the length of the clinical trial period, the number of participants the FDA will require to be enrolled in the trials in order to establish the safety, efficacy, purity and potency of immunotherapy products, or that the data generated in these trials will be acceptable to the FDA to support marketing approval.
Concurrently with clinical trials, companies usually complete additional studies and must also develop additional information about the physical characteristics of the biological product as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. To help reduce the risk of the introduction of adventitious agents with use of biological products, the PHSA emphasizes the importance of manufacturing control for products whose attributes cannot be precisely defined. The manufacturing process must be capable of consistently producing quality batches of the drug candidate and, among other things, the sponsor must develop methods for testing the identity, strength, quality, potency and purity of the final biological product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the biological drug candidate does not undergo unacceptable deterioration over its shelf life.
29
U.S. Review and Approval Processes
Assuming successful completion of the required clinical testing, the results of the preclinical studies and clinical trials, along with information relating to the product’s chemistry, manufacturing, and controls and proposed labeling, are submitted to the FDA as part of a BLA requesting approval to market the product for one or more indications. A BLA in particular must contain proof of the biological product candidate’s safety, purity, potency and efficacy for its proposed indication or indications. Data may come from company-sponsored clinical trials intended to test the safety and efficacy of a product’s use or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and efficacy of the investigational product to the satisfaction of the FDA. The testing and approval processes require substantial time and effort and there can be no assurance that the FDA will accept the BLA for filing and, even if filed, that any approval will be granted on a timely basis, if at all.
Under the Prescription Drug User Fee Act, as amended, or PDUFA, each BLA must be accompanied by a significant user fee, and the sponsor of an approved BLA is also subject to an annual program fee. The FDA adjusts the PDUFA user fees on an annual basis. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application filed by a small business. Additionally, no user fees are assessed on BLAs for products designated as orphan drugs, unless the product also includes a non-orphan indication.
According to the goals and policies for original BLAs agreed to by the FDA under PDUFA, the FDA has ten months from the accepted for filing date in which to complete its initial review of a standard application and respond to the applicant, and six months from the filing date for an application with priority review. For all BLAs, the ten and six-month time periods run from the filing date; for most other original applications, the ten and six-month time periods run from the submission date. Despite these review goals, it is not uncommon for FDA review of a BLA to extend beyond the goal date.
Within 60 days following submission of the application, the FDA reviews a BLA submitted to determine if it is substantially complete before the agency accepts it for filing. The FDA may refuse to file any BLA that it deems incomplete or not properly reviewable at the time of submission and may request additional information. In this event, the BLA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review of the BLA. The FDA reviews the BLA to determine, among other things, whether the proposed product is safe, potent, and/or effective for its intended use, and has an acceptable purity profile, and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, safety, strength, quality, potency and purity. The review process may be extended by the FDA for three additional months to consider new information or in the case of a clarification provided by the applicant to address an outstanding deficiency identified by the FDA following the original submission.
The FDA may refer applications for novel biological products or biological products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making final decisions on approval. The FDA likely will re-analyze the clinical trial data, which could result in extensive discussions between the FDA and the applicant during the review process. The FDA also may require submission of a risk evaluation and mitigation strategy, or REMS, if it determines that a REMS is necessary to ensure that the benefits of the drug outweigh its risks and to assure the safe use of the drug or biological product. The REMS could include medication guides, physician communication plans, assessment plans and/or elements to assure safe use, such as restricted distribution methods, patient registries or other risk minimization tools. The FDA determines the requirement for a REMS, as well as the specific REMS provisions, on a case-by-case basis. If the FDA concludes a REMS is needed, the sponsor of the BLA must submit a proposed REMS. The FDA will not approve a BLA without a REMS, if required.
Before approving a BLA, the FDA will typically conduct a pre-approval inspection of the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. For immunotherapy products, the FDA also will not approve the product if the manufacturer is not in compliance with the cGTPs, to the extent applicable. These are FDA regulations and guidance documents that govern the methods used in, and the facilities and controls used for, the manufacture of human cells, tissues, and cellular and tissue based products (HCT/Ps), which are human cells or tissue intended for implantation, transplant, infusion, or transfer into a human recipient. The primary intent of the cGTP requirements is to ensure that cellular tissue-based products are manufactured in a manner designed to prevent the introduction, transmission and spread of communicable disease. Additionally, before approving a BLA, the FDA will typically inspect one or more clinical sites to assure that the clinical trials were conducted in compliance with IND trial requirements and GCP requirements. To assure cGMP, cGTP and GCP compliance, an applicant must incur significant expenditure of time, money and effort in the areas of training, record keeping, production, and quality control.
30
In addition, under the Pediatric Research Equity Act, or PREA, a BLA or supplement to a BLA must contain data to assess the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may grant deferrals for submission of data or full or partial waivers. A sponsor who is planning to submit a marketing application for a product that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration is required to submit an initial Pediatric Study Plan, or iPSP, within sixty days of an end-of-phase 2 meeting or, if there is no such meeting, as early as practicable before the initiation of the phase 3 or phase 2/3 clinical trial. The iPSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including trial objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. The FDA and the sponsor must reach an agreement on the iPSP. A sponsor can submit amendments to an agreed upon iPSP at any time if changes to the pediatric plan need to be considered based on data collected from preclinical studies, early phase clinical trials or other clinical development programs. Unless otherwise required by regulation, the PREA does not apply to any product for an indication for which orphan designation has been granted. However, if only one indication for a product has orphan designation, a pediatric assessment may still be required for any applications to market that same product for the non-orphan indication(s).
Notwithstanding the submission of relevant data and information, the FDA may ultimately decide that the BLA does not satisfy its regulatory criteria for approval and deny approval or may require additional clinical or other data and information. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than we interpret the same data. On the basis of the FDA’s evaluation of the BLA and accompanying information, including the results of the inspection of the manufacturing facilities, the FDA may issue either an approval letter or a Complete Response Letter, or CRL. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A CRL indicates that the review cycle of the application is complete and the application will not be approved in its present form. A CRL generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. The CRL may require additional clinical or other data, additional pivotal phase 3 clinical trial(s) and/or other significant and time-consuming requirements related to clinical trials, preclinical studies or manufacturing. If a CRL is issued, the applicant may choose to either resubmit the BLA addressing all of the deficiencies identified in the letter, or withdraw the application. If and when those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the BLA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in response to an issued CRL in either two or six months depending on the type of information included. Even with the submission of this additional information, however, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
If a product receives regulatory approval from the FDA, the approval is limited to the conditions of use (e.g., patient population, indication) described in the application. Further, depending on the specific risk(s) to be addressed, the FDA may require that contraindications, warnings or precautions be included in the product labeling, require that post-approval trials, including phase 4 clinical trials, be conducted to further assess a product’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution and use restrictions or other risk management mechanisms under a REMS, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-marketing trials or surveillance programs. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Fast Track, Breakthrough Therapy and Priority Review Designations
The FDA is authorized to designate certain products for expedited development or review if they are intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition. These programs include fast track designation, Breakthrough Therapy Designation and priority review designation and regenerative medicine advanced therapy designation.
To be eligible for a fast track designation, the FDA must determine, based on the request of a sponsor, that a product is intended to treat a serious or life-threatening disease or condition and demonstrates the potential to address an unmet medical need by providing a therapy where none exists or a therapy that may be potentially superior to existing therapy based on efficacy or safety factors. Fast track designation provides opportunities for more frequent interactions with the FDA review team to expedite development and review of the product. The FDA may also review sections of the BLA for a fast track product on a rolling basis before the complete application is submitted, if the sponsor and the FDA agree on a schedule for the submission of the application sections and the sponsor pays any required user fees upon submission of the first section of the NDA or BLA. In addition, fast track designation may be withdrawn by the sponsor or rescinded by the FDA if the designation is no longer supported by data emerging from the clinical trial process.
31
In addition, with the enactment of the Food and Drug Administration Safety and Innovation Act, or FDASIA, in 2012, Congress created a new regulatory program for product candidates designated by FDA as “breakthrough therapies” upon a request made by the IND sponsors. A breakthrough therapy is defined as a drug or biologic that is intended, alone or in combination with one or more other drugs or biologics, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug or biologic may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Drugs or biologics designated as breakthrough therapies are also eligible for accelerated approval of their respective marketing applications. The FDA must take certain actions with respect to breakthrough therapies, such as holding timely meetings with and providing advice to the product sponsor, which are intended to expedite the development and review of an application for approval of a breakthrough therapy.
Next, the FDA may designate a product for priority review if it is a drug or biologic that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The FDA determines at the time that the marketing application is submitted, on a case-by-case basis, whether the proposed drug represents a significant improvement in treatment, prevention or diagnosis of disease when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment-limiting drug reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes, or evidence of safety and effectiveness in a new subpopulation. A priority review designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA’s goal for taking action on a marketing application from ten months to six months for an original BLA from the date of filing.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened. Furthermore, fast track designation, breakthrough therapy designation and priority review do not change the standards for approval and may not ultimately expedite the development or approval process.
As part of the 21st Century Cures Act, congress created an accelerated approval pathway for regenerative medicine advanced therapies, or RMATs, which includes therapeutic tissue engineered products, human cell and tissue products, cell therapies and combination products using any such therapies. The program is intended to facilitate expedited development and review of RMATs intended to address serious diseases or conditions.
A sponsor may request a RMAT designation from the FDA concurrently with or any time after the IND submission. The FDA has 60 calendar days to determine if the drug product meets the required criteria. Preliminary clinical evidence that the product has the potential to address a serious unmet need or condition is expected, is not required to indicate that the drug product may offer significant improvement over current therapies. The RMAT designation provides the same benefits of the fast track and breakthrough designation programs and programs may be eligible for priority review. Products with the RMAT designation may also be eligible for accelerated approval if pre-agreed criteria are met.
Accelerated Approval Pathway
In addition, products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval from the FDA and may be approved on the basis of adequate and well-controlled clinical trials establishing that the drug product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. The FDA may also grant accelerated approval for such a drug or biologic when the product has an effect on an intermediate clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality, or IMM, and that is reasonably likely to predict an effect on IMM or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform post-marketing clinical trials to verify and describe the predicted effect on IMM or other clinical endpoint, and the product may be subject to expedited withdrawal procedures. Drugs and biologics granted accelerated approval must meet the same statutory standards for safety and effectiveness as those granted traditional approval.
For the purposes of accelerated approval, a surrogate endpoint is a marker, such as a laboratory measurement, radiographic image, physical sign, or other measure that is thought to predict clinical benefit, but is not itself a measure of clinical benefit. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. An intermediate clinical endpoint is a measurement of a therapeutic effect that is considered reasonably likely to predict the clinical benefit of a drug, such as an effect on IMM. The FDA has limited experience with accelerated approvals based on intermediate clinical endpoints, but has indicated that such endpoints generally may support accelerated approval when the therapeutic effect measured by the endpoint is not itself a clinical benefit and basis for traditional approval, if there is a basis for concluding that the therapeutic effect is reasonably likely to predict the ultimate long-term clinical benefit of a drug.
32
The accelerated approval pathway is most often used in settings in which the course of a disease is long and an extended period of time is required to measure the intended clinical benefit of a drug, even if the effect on the surrogate or intermediate clinical endpoint occurs rapidly. For example, accelerated approval has been used extensively in the development and approval of drugs for treatment of a variety of cancers in which the goal of therapy is generally to improve survival or decrease morbidity and the duration of the typical disease course requires lengthy and sometimes large clinical trials to demonstrate a clinical or survival benefit.
The accelerated approval pathway is usually contingent on a sponsor’s agreement to conduct, in a diligent manner, additional post-approval confirmatory studies to verify and describe the drug’s clinical benefit. As a result, a product candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or to confirm the predicted clinical benefit of the product during post-marketing studies, would allow the FDA to withdraw approval of the drug. All promotional materials for product candidates being considered and approved under the accelerated approval program are subject to prior review by the FDA.
Orphan Drug Designation and Exclusivity
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug or biologic product intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than
200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug or biologic for this type of disease or condition will be recovered from sales in the United States for that drug or biologic. Orphan drug designation must be requested before submitting an NDA or BLA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use will be disclosed publicly by the FDA; the posting will also indicate whether the drug or biologic is no longer designated as an orphan drug. More than one product candidate may receive an orphan drug designation for the same indication. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to seven years of orphan product exclusivity. During the seven-year exclusivity period, the FDA may not approve any other applications to market a product containing the same active moiety for the same disease, except in very limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. A product is clinically superior if it is safer, more effective or makes a major contribution to patient care. Thus, orphan drug exclusivity could block the approval of one of our potential products for seven years if a competitor obtains approval of the same product as defined by the FDA and we are not able to show the clinical superiority of our product candidate or if our product candidate’s indication is determined to be contained within the competitor’s product orphan indication.
In addition, the FDA will not recognize orphan drug exclusivity if a sponsor fails to demonstrate upon approval that the product is clinically superior to a previously approved product containing the same active moiety for the same orphan condition, regardless of whether or not the approved product was designated an orphan drug or had orphan drug exclusivity.
Patent Term Restoration
Depending upon the timing, duration and specifics of FDA approval of our biological products, some of our US patents may be eligible for limited patent term extension. These patent term extensions permit a patent restoration term of up to five years as compensation for any patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND, and the submission date of a BLA, plus the time between the submission date of a BLA and the approval of that application. Only one patent applicable to an approved biological product is eligible for the extension, and the extension must be applied for prior to expiration of the patent. The United States Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
Pediatric Exclusivity
Pediatric exclusivity is a type of non-patent marketing exclusivity available in the United States and, if granted, it provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity or listed patents. This six-month exclusivity may be granted if a sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The data do not need to show the product to be effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA’s request, the additional protection is granted. If reports of requested pediatric studies are
33
submitted to and accepted by the FDA within the statutory time limits, whatever statutory or regulatory periods of exclusivity or patent protection cover the product are extended by six months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot approve another application. The issuance of a Written Request does not require the sponsor to undertake the described studies.
Reference Product Exclusivity for Biological Products
In March 2010, the Patient Protection and Affordable Care Act was enacted in the United States and included the Biologics Price Competition and Innovation Act of 2009, or the BPCIA. The BPCIA amended the PHSA to create an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. To date, the FDA has approved a number of biosimilars, and numerous biosimilars have been approved in Europe. The FDA has also issued several guidance documents outlining its approach to reviewing and approving biosimilars and interchangeable biosimilars.
A biosimilar product is defined as one that is highly similar to a reference product notwithstanding minor differences in clinically inactive components and for which there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity and potency of the product. An interchangeable product is a biosimilar product that can be expected to produce the same clinical results as the reference product in any given patient and, for products administered multiple times to an individual, that the product and the reference product may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biological product without such alternation or switch. Upon licensure by the FDA, an interchangeable biosimilar may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.
The biosimilar applicant must demonstrate that the product is biosimilar based on data from (i) analytical studies showing that the biosimilar product is highly similar to the reference product; (ii) animal studies (including toxicity); and (iii) one or more clinical studies to demonstrate safety, purity and potency in one or more appropriate conditions of use for which the reference product is approved. In addition, the applicant must show that the biosimilar and reference products have the same mechanism of action for the conditions of use on the label, route of administration, dosage and strength, and the production facility must meet standards designed to assure product safety, purity and potency.
A reference biological product is granted 12 years of regulatory exclusivity from the time of first licensure of the product, and the first approved interchangeable biologic product to a reference product will be granted an exclusivity period of up to one year after it is first commercially marketed. If pediatric studies are performed and accepted by the FDA as responsive to a Written Request, the 12-year exclusivity period will be extended for an additional six months. In addition, the FDA will not accept an application for a biosimilar or interchangeable product based on the reference biological product until four years after the date of first licensure of the reference product. “First licensure” typically means the initial date the particular product at issue was licensed in the United States. Date of first licensure does not include the date of licensure of (and a new period of exclusivity is not available for) a supplement for the reference product for a subsequent application filed by the same sponsor or manufacturer of the reference product (or licensor, predecessor in interest or other related entity) for a change (not including a modification to the structure of the biological product) that results in a new indication, route of administration, dosing schedule, dosage form, delivery system, delivery device or strength or for a modification to the structure of the biological product that does not result in a change in safety, purity or potency. Therefore, one must determine whether a new product includes a modification to the structure of a previously licensed product that results in a change in safety, purity or potency to assess whether the licensure of the new product is a first licensure that triggers its own period of exclusivity. Whether a subsequent application, if approved, warrants exclusivity as the “first licensure” of a biological product is determined on a case-by-case basis with data submitted by the sponsor.
The BPCIA is complex and is still being interpreted and implemented by the FDA. In addition, recent government proposals have sought to reduce the 12-year reference product exclusivity period. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. As a result, the ultimate impact, implementation and meaning of the BPCIA is subject to continued uncertainty.
Post-Approval Requirements
Any potential products for which we receive FDA approvals are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, and complying with FDA promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, restrictions on promoting products for uses or in patient populations that are not described in the product’s approved uses (known as off-label use), limitations on industry-sponsored scientific and educational activities, and requirements for promotional activities involving the internet. Although physicians may prescribe legally available products for off-label uses, if the physicians deem to be appropriate in their professional medical
34
judgment, it is FDA’s position that manufacturers may not market or promote such off-label uses. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability, including liability under federal fraud and abuse and civil and criminal false claims laws. If there are any modifications to the product, including changes in indications, labeling or manufacturing processes or facilities, the applicant may be required to submit and obtain FDA approval of a new BLA or a supplement, which may require the applicant to develop additional data or conduct additional preclinical studies and clinical trials. The FDA may also place other conditions on approvals including the requirement for a REMS to assure the safe use of the product. A REMS could include medication guides, physician communication plans or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of products. Product approvals may be withdrawn for non-compliance with regulatory standards or if problems occur following initial marketing.
In addition, quality control and manufacturing procedures must continue to conform to applicable manufacturing requirements after approval to ensure the quality and long-term stability of the product. We expect to rely on third parties for the production of clinical and commercial quantities of our potential products in accordance with cGMP regulations. The cGMP regulations include requirements relating to organization of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls, records and reports and returned or salvaged products. The manufacturing facilities for our product candidates must meet cGMP requirements and satisfy the FDA or comparable foreign regulatory authorities before any product is approved and our commercial products can be manufactured. We rely, and expects to continue to rely, on third parties for the production of clinical and commercial quantities of our products in accordance with cGMP regulations. These manufacturers must comply with cGMP regulations that require, among other things, quality control and quality assurance, the maintenance of records and documentation and the obligation to investigate and correct any deviations from cGMP. Manufacturers and other entities involved in the manufacture and distribution of approved products are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance. Future inspections by the FDA and other regulatory agencies may identify compliance issues at the facilities of our contract manufacturing organizations, or CMOs, that may disrupt production or distribution or require substantial resources to correct. In addition, the discovery of conditions that violate these rules, including failure to conform to cGMP regulations, could result in enforcement actions, and the discovery of problems with a product after approval may result in restrictions on a product, manufacturer, or holder of an approved BLA, including, among other things, voluntary recall and regulatory sanctions as described below.
Once an approval of a product is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in mandatory revisions to the approved labeling to add new safety information; imposition of post-market or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
35
In addition, the Drug Supply Chain Security Act, or DSCSA, was enacted with the aim of building an electronic system to identify and trace certain prescription drugs distributed in the United States, including most biological products. The DSCSA mandates phased-in and resource-intensive obligations for pharmaceutical manufacturers, wholesale distributors, and dispensers over a 10-year period that has been extended an additional year to be implemented in November 2024. In the Fall of 2024, the FDA granted an additional extension to 2025 based on the type of activities being performed. From time to time, new legislation and regulations may be implemented that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA. It is impossible to predict whether further legislative or regulatory changes will be enacted, or FDA regulations, guidance or interpretations changed or what the impact of such changes, if any, may be.
Regulation Outside of the United States
In addition to regulations within the United States, we will be subject to a variety of foreign regulations governing clinical trials and the commercial sale and distribution of our products outside of the United States. Whether or not we obtain FDA approval for a product candidate, we must obtain approval by the comparable regulatory authorities of foreign countries or economic areas, such as the 27-member European Union, before we may commence clinical trials or market products in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly between countries and jurisdictions and can involve additional testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
European Union drug development, review and approval
In the European Union, our product candidates also may be subject to extensive regulatory requirements. As in the United States, medicinal products can be marketed only if a marketing authorization from the competent regulatory agencies has been obtained. Similar to the United States, the various phases of preclinical and clinical research in the European Union are subject to significant regulatory controls.
The Clinical Trials Directive 2001/20/EC, the Directive 2005/28/EC on GCP, and the related national implementing provisions of the individual EU Member States govern the system for the approval of clinical trials in the European Union. Under this system, an applicant must obtain prior approval from the competent national authority of the EU Member States in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial at a specific study site after the competent ethics committee has issued a favorable opinion. The clinical trial application must be accompanied by, among other documents, an IMPD (the Common Technical Document) with supporting information prescribed by Directive 2001/20/EC, Directive 2005/28/EC, where relevant the implementing national provisions of the individual EU Member States and further detailed in applicable guidance documents. All suspected unexpected serious adverse reactions to the investigated drug that occur during the clinical trial have to be reported to the competent national authority and the Ethics Committee of the Member State where they occurred.
In April 2014, the new Clinical Trials Regulation, (EU) No 536/2014 (Clinical Trials Regulation) was adopted and came into application in January 2022. The Clinical Trials Regulation is directly applicable in all the EU Member States, repealing the prior Clinical Trials Directive 2001/20/EC.
The new Clinical Trials Regulation simplifies and streamlines the approval of clinical trials in the European Union. The main characteristics of the regulation include: a streamlined application procedure via a single entry point, the “EU portal”; a single set of documents to be prepared and submitted for the application as well as simplified reporting procedures for clinical trial sponsors; and a harmonized procedure for the assessment of applications for clinical trials, which is divided in two parts. Part I is assessed by the competent authorities of all EU Member States in which an application for authorization of a clinical trial has been submitted (Member States concerned). Part II is assessed separately by each Member State concerned. Strict deadlines have been established for the assessment of clinical trial applications. The role of the relevant ethics committees in the assessment procedure will continue to be governed by the national law of the concerned EU Member State. However, overall related timelines will be defined by the Clinical Trials Regulation.
To obtain a marketing authorization of a drug in the European Union, we may submit marketing authorization applications, or MAA, either under the so-called centralized or national authorization procedures.
36
Centralized Procedure
The centralized procedure provides for the grant of a single marketing authorization following a favorable opinion by the European Medicines Agency, or EMA, that is valid in all 27 European Union member states, or EU member states, as well as Iceland, Liechtenstein and Norway. The centralized procedure is compulsory for medicines produced by specified biotechnological processes, products designated as orphan medicinal products, advanced-therapy medicines (such as gene-therapy, somatic cell-therapy or tissue-engineered medicines) and products with a new active substance indicated for the treatment of specified diseases, such as HIV/AIDS, cancer, diabetes, neurodegenerative disorders or autoimmune diseases and other immune dysfunctions and viral diseases. The centralized procedure is optional for products that represent a significant therapeutic, scientific or technical innovation, or whose authorization would be in the interest of public health. Under the centralized procedure the maximum timeframe for the evaluation of an MAA by the EMA is 210 days, excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the Committee for Medicinal Products for Human Use, or the CHMP. Accelerated assessment might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest, particularly from the point of view of therapeutic innovation. The timeframe for the evaluation of an MAA under the accelerated assessment procedure is of 150 days, excluding stop-clocks.
National authorization procedures
There are also two other possible routes to authorize medicinal products in several EU countries, which are available for investigational medicinal products that fall outside the scope of the centralized procedure:
Under the above-described procedures, before granting the marketing authorization, the EMA or the competent authorities of the Member States of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
Conditional Approval
In specific circumstances, E.U. legislation (Article 14(7) Regulation (EC) No 726/2004 and Regulation (EC) No 507/2006 on Conditional Marketing Authorizations for Medicinal Products for Human Use) enables applicants to obtain a conditional marketing authorization prior to obtaining the comprehensive clinical data required for an application for a full marketing authorization. Such conditional approvals may be granted for product candidates (including medicines designated as orphan medicinal products) if (i) the risk-benefit balance of the product candidate is positive, (ii) it is likely that the applicant will be in a position to provide the required comprehensive clinical trial data, (iii) the product fulfills unmet medical needs and (iv) the benefit to public health of the immediate availability on the market of the medicinal product concerned outweighs the risk inherent in the fact that additional data are still required. A conditional marketing authorization may contain specific obligations to be fulfilled by the marketing authorization holder, including obligations with respect to the completion of ongoing or new studies, and with respect to the collection of pharmacovigilance data. Conditional marketing authorizations are valid for one year, and may be renewed annually, if the risk-benefit balance remains positive, and after an assessment of the need for additional or modified conditions or specific obligations. The timelines for the centralized procedure described above also apply with respect to the review by the CHMP of applications for a conditional marketing authorization.
Pediatric Studies
Prior to obtaining a marketing authorization in the European Union, applicants have to demonstrate compliance with all measures included in an EMA-approved Pediatric Investigation Plan, or PIP, covering all subsets of the pediatric population, unless the EMA has granted a product-specific waiver, a class waiver, or a deferral for one or more of the measures included in the PIP. The respective requirements for all marketing authorization procedures are set forth in Regulation (EC) No 1901/2006, which is referred to as the Pediatric Regulation. This requirement also applies when a company wants to add a new indication, pharmaceutical form or route of administration for a medicine that is already authorized. The Pediatric Committee of the EMA, or PDCO, may grant deferrals for some medicines, allowing a company to delay development of the medicine in children until there is enough information to demonstrate its effectiveness and safety in adults. The PDCO may also grant waivers when development of a medicine in children is not needed or is not appropriate, such as for diseases that only affect the elderly population.
37
Before a marketing authorization application can be filed, or an existing marketing authorization can be amended, the EMA determines that companies actually comply with the agreed studies and measures listed in each relevant PIP.
European Union Regulatory Exclusivity
In the European Union, new products authorized for marketing (i.e., reference products) qualify for eight years of data exclusivity and an additional two years of market exclusivity upon marketing authorization. The data exclusivity period prevents generic or biosimilar applicants from relying on the preclinical and clinical trial data contained in the dossier of the reference product when applying for a generic or biosimilar marketing authorization in the European Union during a period of eight years from the date on which the reference product was first authorized in the European Union. The market exclusivity period prevents a successful generic or biosimilar applicant from commercializing its product in the EU until ten years have elapsed from the initial authorization of the reference product in the EU. The ten-year market exclusivity period can be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. In 2024, the European Parliament voted to adopt a new draft Regulation and draft Directive from the European Commission. These proposals set out significant amendments to the rules regarding regulatory data exclusivity and market protection for new medicines in Europe. The draft legislation will need to be approved by the European Council, before implementation in the EU. Once implemented, the Regulation will take effect in all EU Member States on a defined date, likely to be approximately in three to four years.
European Union Orphan Designation and Exclusivity
The criteria for designating an orphan medicinal product in the European Union, are similar in principle to those in the United States. Under Article 3 of Regulation (EC) 141/2000, a medicinal product may be designated as orphan if (i) it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition; (ii) either (a) such condition affects no more than five in 10,000 persons in the European Union when the application is made, or (b) the product, without the benefits derived from orphan status, would not generate sufficient return in the European Union to justify investment; and (iii) there exists no satisfactory method of diagnosis, prevention or treatment of such condition authorized for marketing in the European Union, or if such a method exists, the product will be of significant benefit to those affected by the condition, as defined in Regulation (EC) 847/2000. Orphan medicinal products are eligible for financial incentives such as reduction of fees or fee waivers and are, upon grant of a marketing authorization, entitled to ten years of market exclusivity for the approved therapeutic indication. The application for orphan designation must be submitted before the application for marketing authorization. The applicant will receive a fee reduction for the marketing authorization application if the orphan designation has been granted, but not if the designation is still pending at the time the marketing authorization is submitted. Orphan designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
The ten-year market exclusivity in the European Union may be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan designation, for example, if the product is sufficiently profitable not to justify maintenance of market exclusivity. Additionally, marketing authorization may be granted to a similar product for the same indication at any time if:
PRIME Designation
The EMA grants access to the Priority Medicines, or PRIME, program to investigational medicines for which it determines there to be preliminary data available showing the potential to address an unmet medical need and bring a major therapeutic advantage to patients. As part of the program, EMA provides early and enhanced dialogue and support to optimize the development of eligible medicines and speed up their evaluation, aiming to bring promising treatments to patients sooner.
38
Periods of Authorization and Renewals
A marketing authorization is valid for five years in principle and the marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the authorizing member state. To this end, the marketing authorization holder must provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases to be valid. Once renewed, the marketing authorization is valid for an unlimited period, unless the European Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal. Any authorization which is not followed by the actual placing of the drug on the EU market (in case of centralized procedure) or on the market of the authorizing member state within three years after authorization ceases to be valid (the so-called sunset clause).
Rest of the World Regulation
For other countries outside of the European Union and the United States, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from jurisdiction to jurisdiction. Additionally, the clinical trials must be conducted in accordance with GCP requirements and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Coverage, Pricing and Reimbursement
Sales of pharmaceutical products approved by the FDA will depend, in significant part, on the availability of third-party coverage and reimbursement for the products. Third-party payors include government healthcare programs in the United States such as Medicare and Medicaid, managed care providers, private health insurers and other organizations. These third-party payors are increasingly challenging the prices of products and examining the cost-effectiveness of medical products and services. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare products. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Further, there is no uniform policy for coverage and reimbursement in the United States by third-party payors. Third-party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the approved products for a particular indication. We may need to conduct expensive pharmacoeconomic studies to demonstrate the medical necessity and cost-effectiveness of our products, in addition to the costs required to obtain FDA or other comparable regulatory approvals.
Moreover, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Third-party reimbursement may not be sufficient to maintain price levels high enough to realize an appropriate return on investment in product development. Our product candidates may not be considered cost-effective. It is time consuming and expensive to seek coverage and reimbursement from third-party payors. Coverage and reimbursement may not be available or sufficient to allow us to sell our products on a competitive and profitable basis.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of our product candidate to currently available therapies (so called health technology assessment, or HTA) in order to obtain reimbursement or pricing approval. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. An EU Member State may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the ompany placing the medicinal product on the market. Other EU Member States allow companies to fix their own prices for drug products but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the United States and generally tend to be significantly lower.
39
The downward pressure on health care costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various EU Member States and parallel distribution (arbitrage between low-priced and high- priced member states) can further reduce prices. Any country that has price controls or reimbursement limitations for drug products may not allow favorable reimbursement and pricing arrangements.
Other U.S. Health Care Laws and Regulations
Although we currently do not have any products on the market, our current and future arrangements with healthcare professionals, investigators, consultants, customers and third-party payors expose us to broadly applicable healthcare regulation and enforcement by the U.S. federal government and the states and foreign governments in which we conducts our business, such as fraud and abuse laws, transparency and health information privacy rules and regulations. These laws include, without limitation:
40
41
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines, such as the PhRMA Code, or the relevant compliance guidance promulgated by the federal government, in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures to the extent that those laws impose requirements that are more stringent than the Physician Payments Sunshine Act. In addition, state and local laws may require the registration of pharmaceutical sales representatives. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Violations of any of such laws or any other governmental regulations that apply to us, may subject us to significant penalties, including, without limitation, civil, criminal and administrative penalties, damages, fines, disgorgement, additional reporting requirements and oversight if the Company becomes subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect our ability to operate our business.
Health Care Reform in the United States and Potential Changes to Health Care Laws
The FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we otherwise may have obtained and may not achieve or sustain profitability, which would adversely affect our business, prospects, financial condition and results of operations.
As previously mentioned, a primary trend in the U.S. health care industry and elsewhere is cost containment. Government authorities and other third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medical products and services, implementing reductions in Medicare and other health care funding and applying new payment methodologies. For example, in March 2010, the ACA was enacted, which, among other things, increased the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program; introduced a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected; extended the Medicaid Drug Rebate Program to utilization of prescriptions of individuals enrolled in Medicaid managed care plans; imposed mandatory discounts for certain Medicare Part D beneficiaries as a condition for manufacturers’ outpatient drugs coverage under Medicare Part D; and established a Center for Medicare Innovation at CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA that affect health care expenditures. There has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries, presidential executive orders and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for pharmaceutical and biologic products. Notably, on December 20, 2019, President Trump signed the Further Consolidated Appropriations Act for 2020 into law (P.L. 116-94) that includes a piece of bipartisan legislation called the Creating and Restoring Equal Access to Equivalent Samples Act of 2019 or the “CREATES Act.” The CREATES Act aims to address the concern articulated by both the FDA and others in the industry that some brand manufacturers have improperly restricted the distribution of their products, including by invoking the existence of a REMS for certain products, to deny generic and biosimilar product developers access to samples of brand products. Because generic and biosimilar product developers need samples to conduct certain comparative testing required by the FDA, some have attributed the inability to timely obtain samples as a cause of delay in the entry of generic and biosimilar products. To remedy this concern, the CREATES Act establishes a private cause of action that permits a generic or biosimilar product developer to sue the brand manufacturer to compel it to furnish the necessary samples on “commercially reasonable, market-based terms.” Whether and how generic and biosimilar product developments will use this new pathway, as well as the likely outcome of any legal challenges to provisions of the CREATES Act, remain highly uncertain and its potential effects on our future commercial products are unknown. The FDA also released a final rule on September 24, 2020 providing guidance for states to build and submit importation plans for drugs from Canada. This final rule became effective November 30, 2020. In January 2024, FDA authorized the state of Florida’s Section 804 Importance Program to allow Florida to import drugs from Canada for a period of two years. The ongoing impact of this and potentially other state programs is still unclear.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. It is also possible that additional governmental action is taken in response to the COVID-19 pandemic. We expect that additional state and federal health care reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for health care products and services.
42
Facilities
Our principal office is located in Tampa, Florida. We currently lease approximately 12,199 square feet of office and laboratory space under a lease that is due to expire in March 2026. We believe that such office and laboratory space will be sufficient for our planned operations for the foreseeable future.
Corporate Information
Our principal executive offices are located at 10500 University Center Drive, Suite 110, Tampa, Florida 33612. Our telephone number is (813) 875-6600. Our principal website address is www.tuhurabio.com. Information contained on or accessible through our website is not a part of this Annual Report on Form 10-K, and the inclusion of our website address in this Annual Report on Form 10-K is for convenience only and the information on the referenced website does not constitute a part of nor is incorporated by reference into this report.
Our reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, including our annual reports on Form 10-K, our quarterly reports on Form 10-Q and our current reports on Form 8-K, and amendments to those reports, are accessible through our website, free of charge, as soon as reasonably practicable after these reports are filed electronically with, or otherwise furnished to, the SEC. These SEC reports can be accessed through the “Investors” section of our website.
Item 1A. Risk Factors.
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, as well as the other information in this Annual Report, including our audited financial statements and the related notes, as well as our other public filings with the SEC, before deciding to invest in our common stock. If any of the following risks are realized, our business, financial condition, results of operations and prospects, as well as the price of our common stock, could be materially and adversely affected.
SUMMARY OF RISK FACTORS
43
An investment in our common stock involves a high degree of risk. In determining whether to purchase our common stock, an investor should carefully consider all of the material risks described below, together with the other information contained in this Annual Report before making a decision to purchase our securities. An investor should only purchase our securities if he, she or it can afford to suffer the loss of his, her or its entire investment.
Risks Relating to Our Business and Industry
We are a clinical-stage company and has a limited operating history, which may make it difficult to evaluate our current business and predict our future performance.
We are a clinical-stage pharmaceutical company and have no products approved for commercial sale. We employ a multi-indication immunomodulator platform (ImmuneFx) that utilizes both cell and gene therapies, together, to stimulate the immune system to recognize and combat tumor cells. Although there have been significant advances in cell and gene-based immunotherapies, our immunomodulatory platforms are new and largely unproven. Our operations to date have been limited to organizing and staffing the Company, business planning, raising capital, developing our technology, identifying potential product candidates, undertaking preclinical studies, and conducting clinical trials. If one of our product candidates received regulatory approval, we would need to transition from a company with a research and development focus to a company capable of supporting commercial activities. We may not be successful in such a transition. In addition, our limited operating history, particularly in light of the rapidly evolving cancer immunotherapy field, may make it difficult to evaluate our current business and predict our future performance. We will encounter risks and difficulties frequently experienced by early-stage companies in rapidly evolving fields. If it does not address these risks successfully, our business will suffer.
Legacy TuHURA, our main operating subsidiary, has incurred significant losses since inception and expects to incur significant losses for the foreseeable future and may not be able to achieve or sustain profitability in the future.
Legacy TuHURA is not profitable and has incurred significant losses in each period since its inception, including net losses of $22.6 million for the year ended December 31, 2024, and $29.3 million for the year ended December 31, 2023 (which includes the expensing of the entire $16.2 million purchase price for the assets of TuHURA Biopharma, of which $15.0 million was paid in the form of our common stock). To date, we have financed our operations primarily through private placements of our preferred stock and convertible notes. We have not commercialized any products and has never generated any revenue from product sales. We expect these losses to increase as it continues to incur significant research and development and other expenses related to our ongoing operations, seeks regulatory approvals for our product candidates, scales-up manufacturing capabilities and hires additional personnel to support the development of our product candidates and to enhance our operational, financial and information management systems.
44
A critical aspect of our strategy is to invest significantly in our technology platform to improve the efficacy and safety of our product candidates. To become and remain profitable, we must develop and eventually commercialize products with significant market potential, which it may never achieve. Even if we succeed in commercializing one or more of these product candidates, we will continue to incur losses for the foreseeable future relating to our substantial research and development expenditures to develop our technologies. We may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. The size of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenue. Our prior losses and expected future losses have had and will continue to have an adverse effect on our stockholders’ equity and working capital. Further, the net losses we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. If we do not achieve profitability, it may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of the Company and could impair our ability to raise capital, maintain our discovery and preclinical and clinical development efforts, expand our business or continue our operations and may require us to raise additional capital that may dilute your ownership interest. A decline in the value of our could also cause you to lose all or part of your investment.
Our recurring losses from operations and financial condition raise substantial doubt about our ability to continue as a going concern.
Our recurring losses from operations and financial condition raise substantial doubt about our ability to continue as a going concern. In our financial statements for the years ended December 31, 2024 and 2023, we concluded that our recurring losses from operations and need for additional financing to fund future operations raise substantial doubt about our ability to continue as a going concern. Similarly, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements for the year ended December 31, 2024 with respect to this uncertainty. Our ability to continue as a going concern will require us to obtain additional funding. If we are unable to obtain sufficient funding, our business, prospects, financial condition and results of operations will be materially and adversely affected, and we may be unable to continue as a going concern. If we are unable to raise capital when needed or on acceptable terms, we would be forced to delay, limit, reduce or terminate our product development or future commercialization efforts of one or more of our product candidates, or may be forced to reduce or terminate our operations. If we are unable to continue as a going concern, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our audited financial statements, and it is likely that investors will lose all or part of their investment. If we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue as a going concern, investors and other financing sources may be unwilling to provide additional funding to it on commercially reasonable terms, if at all.
We have never generated any revenue from product sales for our human drug candidates and our ability to generate revenue from product sales and become profitable depends significantly on our success in numerous endeavors.
We have no products approved for commercial sale, has not generated any revenue from product sales, and does not anticipate generating any revenue from product sales until sometime after we have received regulatory approval for the commercial sale of a product candidate. Our ability to generate revenue and achieve profitability depends significantly on our success in many endeavors, including:
45
Because of the numerous risks and uncertainties associated with biopharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. If we are required by the U.S. Food and Drug Administration (the “FDA”), or other regulatory agencies, domestic or foreign, or other comparable foreign authorities, to perform preclinical studies or clinical trials in addition to those we currently anticipate, or if there are any delays in completing our clinical trials or the development of any of our product candidates, our expenses could increase and revenue could be further delayed.
Even if one or more of the product candidates that we develop is approved for commercial sale, we anticipate incurring significant costs associated with commercializing any approved product candidate. Our expenses could increase beyond expectations if we are required by the FDA or other regulatory agencies, domestic or foreign, to change our manufacturing processes or assays, or to perform clinical, nonclinical, or other types of studies in addition to those that we currently anticipate. If we are successful in obtaining regulatory approvals to market of one or more of our product candidates, our revenue will be dependent, in part, upon the size of the markets in the territories for which we gain regulatory approval, the accepted price for the product, the ability to get reimbursement at any price, and whether we own the commercial rights for that territory. If the number of our addressable disease patients is not as significant as it estimates, the indication approved by regulatory authorities is narrower than it expects, or the reasonably accepted population for treatment is narrowed by competition, physician choice or treatment guidelines, we may not generate significant revenue from sales of such products, even if approved. If we are not able to generate revenue from the sale of any approved products, we may never become profitable.
We will require substantial additional capital to finance our operations in the future. If we fail to obtain additional financing on acceptable terms or at all, we may be unable to complete the development and commercialization of our product candidates.
Our operations have required substantial amounts of cash since inception. We expect to continue to spend substantial amounts to continue the clinical development of our product candidates, particularly as we advance the development of our lead product candidate Ifx-Hu2.0 as a potential treatment for patients with melanoma, bladder and cervical cancers. If we obtain orphan drug designation and marketing approval for Ifx-Hu2.0 or any of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution.
As of December 31, 2024, we had cash and cash equivalents of $12.7 million. Based on our current operating plan, we believe that our existing cash, cash equivalents and short-term investments, together with the anticipated payment of notes receivable from warrant exercises, should be sufficient to fund our operations through late fourth quarter of 2025. This estimate is based on assumptions that may prove to be materially wrong, and we could use our available capital resources sooner than it currently expects because of circumstances beyond our control. We may require additional capital for the further development and commercialization of our product candidates and may need to raise additional funds sooner if we choose to pursue additional indications or geographies for our product candidates or otherwise expand more rapidly than we presently anticipate. Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates.
We cannot be certain that additional funding will be available on acceptable terms, or at all. Our ability to raise additional funding will depend on financial, economic and market conditions and other factors, over which we may have no or limited control. In addition, our ability to obtain future funding when needed through equity financings, debt financings or strategic collaborations may be particularly challenging in light of the uncertainties and circumstances resulting from the ongoing military conflict between Russian and Ukraine, as well as the ongoing conflict between Israel and Hamas, and the global impacts of such conflicts. We have no committed source of additional capital and if we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of our product candidates or other research and development initiatives. Our license and collaboration agreements may also be terminated if we are unable to meet the payment obligations under the agreements. We could be required to seek collaborators for our product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available or relinquish or license on unfavorable terms our rights to our product candidates in markets where we otherwise would seek to pursue development or commercialization ourself.
46
Any of the above events could significantly harm our business, prospects, financial condition, and results of operations and cause the price of shares of our common stock to decline.
The biotechnology and immunotherapy industries are characterized by rapid technological developments and a high degree of competition. we may be unable to compete with more substantial enterprises.
The biotechnology and biopharmaceutical industries are characterized by rapid technological developments and a high degree of competition. As a result, our actual or proposed immunotherapies could become obsolete before we recoup any portion of our related research and development and commercialization expenses. Competition in the biopharmaceutical industry is based significantly on scientific and technological factors. These factors include the availability of patent and other protection for technology and products, the ability to commercialize technological developments and the ability to obtain governmental approval for testing, manufacturing, and marketing. We compete with specialized biopharmaceutical firms in the United States, Europe and elsewhere, as well as a growing number of large pharmaceutical companies that are applying biotechnology to their operations. Many biopharmaceutical companies have focused their development efforts in the human therapeutics area, including cancer. Many major pharmaceutical companies have developed or acquired internal biotechnology capabilities or made commercial arrangements with other biopharmaceutical companies. These companies, as well as academic institutions, governmental agencies and private research organizations, also compete with us in recruiting and retaining highly qualified scientific personnel and consultants. Our ability to compete successfully with other companies in the pharmaceutical field will also depend to a considerable degree on the continuing availability of capital to us.
We are aware of certain investigational new drugs under development or approved products by competitors that are used for the prevention, diagnosis, or treatment of certain diseases we have targeted for drug development. Various companies are developing biopharmaceutical products that have the potential to directly compete with our immunotherapies even though their approach may be different. The competition comes from both biotechnology firms and from major pharmaceutical companies. Many of these companies have substantially greater financial, marketing, and human resources than us. We also experience competition in the development of our immunotherapies from universities, other research institutions and others in acquiring technology from such universities and institutions.
In addition, certain of our immunotherapies may be subject to competition from investigational new drugs and/or products developed using other technologies, some of which have completed numerous clinical trials.
The successful development of immunotherapies is highly uncertain.
Successful development of biopharmaceuticals is highly uncertain and depends on numerous factors, many of which are beyond our control. Immunotherapies that appear promising in the early phases of development may fail to reach the market for several reasons including:
Success in preclinical and early clinical studies does not ensure that large-scale clinical studies will be successful. Clinical results are frequently susceptible to varying interpretations that may delay, limit, or prevent regulatory approvals. The length of time necessary to complete clinical studies and to submit an application for marketing approval for a final decision by a regulatory authority varies significantly from one immunotherapy to the next and may be difficult to predict. The evidence of clinical response rates received to date for Ifx-2.0, our principal product candidate, as well as the other clinical activity and results described in this Report, does not mean that Ifx-2.0 or any other product candidate has demonstrated, or that such clinical response data will predict, sufficient clinical efficacy and prove the required level of safety in order to receive FDA approval or any other required regulatory approval.
47
In addition, we have entered into a Special Protocol Assessment (“SPA”) agreement with the FDA regarding the initiation of a single registration-directed trial utilizing the FDA’s accelerated approval pathway for Ifx-2.0. An SPA agreement for such a trial does not increase the likelihood of marketing approval for the product and may not lead to a faster or less costly development, review, or approval process.
Even if we are successful in getting market approval, commercial success of any of our product candidates will also depend in large part on the availability of coverage and adequate reimbursement from third-party payors, including government payors such as the Medicare and Medicaid programs and managed care organizations, which may be affected by existing and future health care reform measures designed to reduce the cost of health care. Third-party payors could require us to conduct additional studies, including post-marketing studies related to the cost effectiveness of a product, to qualify for reimbursement, which could be costly and divert our resources. If government and other health care payors were not to provide adequate coverage and reimbursement levels for any of our products once approved, market acceptance and commercial success would be reduced.
Our technology platforms, including our proprietary, multi-indication immunomodulatory platform (ImmuneFx Ifx, and Delta receptor targeting ADCs) technologies are a new approach to treat cancer and other immune-related diseases that present significant challenges.
We have concentrated our research and development efforts on advancing a new generation of immunotherapies based on the Ifx and Delta receptor antibody drug conjugates (“ADC”) platforms, and our future success is highly dependent on the successful development of our product candidates, which target cancer and other immune-related diseases. We cannot be sure that our Ifx or Delta receptor ADC platforms will yield satisfactory products that are safe and effective, scalable, or profitable.
Our technology could become subject to many of the challenges and risks that gene therapies face, including:
Moreover, public perception of therapy safety issues, including adoption of new therapeutics or novel approaches to treatment, may adversely influence the willingness of subjects to participate in clinical trials, or if approved, of physicians to subscribe to the novel treatment mechanics. Physicians, hospitals and third-party payors often are slow to adopt new products, technologies and treatment practices that require additional upfront costs and training. Physicians may not be willing to undergo training to adopt this novel and personalized therapy, may decide the therapy is too complex to adopt without appropriate training and may choose not to administer the therapy. Based on these and other factors, hospitals and payors may decide that the benefits of this new therapy do not or will not outweigh its costs.
Our near-term ability to generate product revenue is dependent on the success of one or more of our product candidates, each of which are at an early stage of development and will require significant additional clinical testing before we can seek regulatory approval and begin commercial sales.
Our near-term ability to generate product revenue is highly dependent on our ability to obtain regulatory approval of and successfully commercialize one or more of our product candidates. IFx-2.0 and IFx-Hu3.0 are in late and early stages, respectively, of development and will require additional clinical and nonclinical development, regulatory review, and approval in each jurisdiction in which we intend to market the products, substantial investment, access to sufficient commercial manufacturing capacity, and significant marketing efforts before we can generate any revenue from product sales. Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct extensive clinical trials to demonstrate the safety, purity, and potency of the product candidates in humans. We cannot be certain that any of our product candidates will be successful in clinical trials and they may not receive regulatory approval even if they are successful in clinical trials.
48
Before we can generate any revenues from sales of our lead product candidates, we must complete the following activities for each of them, any one of which it may not be able to successfully complete:
If we are unable to address one or more of these factors in a timely manner or at all, we could experience significant delays in the successful commercialization of, or an inability to successfully commercialize, our product candidates, which would materially harm our business. If we do not receive regulatory approvals for one or more of our product candidates, we may not be able to continue our operations. Even if we successfully obtains regulatory approvals to manufacture and market our product candidates, our revenues will be dependent, in part, upon the size of the markets in the territories for which it gains regulatory approval and has commercial rights. If the markets for patient subsets that we are targeting are not as significant as we estimate, we may not generate significant revenues from sales of such products, if approved.
We may encounter substantial delays in our clinical trials or may not be able to conduct our trials on the timelines we expect.
Clinical testing is expensive, time consuming, and subject to uncertainty. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical trials can occur at any stage of testing, and our future clinical trials may not be successful. Events that may prevent successful or timely completion of clinical development include:
49
50
For example, our IND for our planned Phase 3 trial for IFx-2.0 contemplated by our SPA agreement with the FDA is subject to a partial clinical trial hold as described in a January 2024 letter from the FDA that relates to certain CMC matters for the trial. A partial clinical hold means that the FDA suspends part of the clinical work requested under an IND (i.e., a specific protocol or part of a protocol is not allowed to proceed). The partial hold required us to provide additional CMC information from our contract manufacturers for the Phase 3 trial, complete and qualify a potency assay, and qualify the mixing process for IFx-2.0 at the clinical site prior to initiating the trial. We have reached agreement with FDA on the requirements for lifting the partial clinical hold and believe we will meet the requirements, but there is no assurance that we will be able to complete these requirements on a timely basis, which could delay our expected timetable to complete the trial, or if we are unable to complete these requirements, we will not be able to proceed with the trial.
Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of marketing approval for our product candidates.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to generate revenue. In addition, if we make manufacturing or formulation changes to our product candidates, we may be required to, or it may elect to, conduct additional trials to bridge our modified product candidates to earlier versions. Clinical trial delays could also shorten any periods during which our products have patent protection and may allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates and may harm our business and results of operations.
If we do not achieve our projected development and commercialization goals in accordance with our expected and announced timeframes, the commercialization of any of our product candidates may be delayed, and our business will be harmed.
Elsewhere in this Annual Report, we have provided timing estimates regarding the initiation of clinical trials and clinical development milestones, and the expected availability of data resulting from these trials for certain of our product candidates. We expect to continue to estimate the timing of these types of development milestones and our expected timing for the accomplishment of various other scientific, clinical, regulatory, and other product development objectives. From time to time, we may publicly announce the expected timing of some of these events. However, the achievement of many of these milestones and events may be outside of our control. These timing estimations are based on a variety of assumptions we make, which may cause the actual timing of these events to differ from the timing it expects, including:
If we fail to timely achieve announced milestones, the commercialization of any of our product candidates may be delayed, and our business and results of operations may be harmed.
51
Failure to successfully identify, develop, and commercialize additional therapeutics or product candidates could impair our ability to grow.
Although a substantial amount of our efforts will focus on the continued preclinical and clinical testing and potential approval of the product candidates in our current pipeline, we expect to continue to innovate and potentially expand our portfolio. Research programs to identify product candidates may require substantial additional technical, financial, and human resources and may not result in any new potential product candidates being identified. our success may depend, in part, upon our ability to identify, select, and develop promising product candidates and therapeutics. We may expend resources and ultimately fail to discover and generate additional product candidates suitable for further development. All product candidates are prone to risks of failure typical of biotechnology product development, including the possibility that a product candidate may not be suitable for clinical development due to its harmful side effects, limited efficacy, or other characteristics indicating that it is unlikely to receive approval by the FDA, the EMA, and other comparable foreign regulatory authorities and achieve market acceptance. If we do not successfully develop and commercialize new product candidates we have identified and explored, our business, prospects, financial condition, and results of operations could be adversely affected.
The FDA or comparable foreign regulatory authorities may disagree with our regulatory plans and we may fail to obtain regulatory approval of our product candidates.
The FDA standard for regular approval of a biologic generally requires two well-controlled phase 3 studies or one large and robust, well-controlled phase 3 study in the patient population being studied that provides substantial evidence that a biologic is safe and effective for its proposed indication. Phase 3 clinical trials typically involve hundreds of patients, have significant costs, and take years to complete. Product candidates studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may be eligible for accelerated approval and may be approved on the basis of adequate and well-controlled clinical trials establishing that the product candidate has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. As a condition of accelerated approval, the FDA may require a sponsor of a drug or biologic receiving accelerated approval to perform post-marketing studies to verify and describe the predicted effect on irreversible morbidity or mortality or other clinical endpoint, and the drug or biologic may be subject to withdrawal procedures by the FDA that are more accelerated than those available for regular approvals. Recently, we entered into a SPA agreement with the FDA for a single Phase 3 randomized placebo and injection controlled trial for IFx-2.0, which we believe will lead to initiation of the Phase 3 study in of the second quarter of 2025. If our efforts to obtain approval for IFx-2.0 or any other product candidate is not successful, then we may be required to conduct additional clinical trials beyond those we contemplate, which would likely result in a longer time period to potential approval and commercialization of such product candidate (if approved) and would likely increase the cost of development of such product candidate, all of which could harm the Company’s competitive position in the marketplace and shorten the remaining term of applicable patent coverage after product approval.
As part of its marketing authorization process, the EMA may grant marketing authorizations on the basis of less complete data than is normally required, when, for certain categories of medicinal products, doing so may meet unmet medical needs of patients and serve the interest of public health. In such cases, it is possible for the Committee for Medicinal Products for Human Use (“CHMP”), to recommend the granting of a marketing authorization, subject to certain specific obligations to be reviewed annually, which is referred to as a conditional marketing authorization. This may apply to medicinal products for human use that fall under the jurisdiction of the EMA, including those that aim at the treatment, the prevention, or the medical diagnosis of seriously debilitating diseases or life-threatening diseases and those designated as orphan medicinal products.
A conditional marketing authorization may be granted when the CHMP finds that, although comprehensive clinical data referring to the safety and efficacy of the medicinal product have not been supplied, all the following requirements are met:
52
The granting of a conditional marketing authorization is restricted to situations in which only the clinical part of the application is not yet fully complete. Incomplete nonclinical or quality data may only be accepted if duly justified and only in the case of a product intended to be used in emergency situations in response to public- health threats.
Conditional marketing authorizations are valid for one year, on a renewable basis. The holder will be required to complete ongoing studies or to conduct new studies with a view to confirming that the benefit-risk balance is positive. In addition, specific obligations may be imposed in relation to the collection of pharmacovigilance data.
The granting of a conditional marketing authorization will allow medicines to reach patients with unmet medical needs earlier than might otherwise be the case and will ensure that additional data on a product are generated, submitted, assessed, and acted upon. Although we may seek a conditional marketing authorization for one or more of our product candidates by the EMA, the EMA or CHMP may ultimately not agree that the requirements for such conditional marketing authorization have been satisfied.
Our clinical trial results may also not support approval, whether accelerated approval, conditional marketing authorizations, or regular approval. The results of preclinical studies and clinical trials may not be predictive of the results of later-stage clinical trials, and product candidates in later stages of clinical trials may fail to show the desired safety and efficacy despite having progressed through preclinical studies and initial clinical trials. In addition, our product candidates could fail to receive regulatory approval for many reasons, including the following:
Further, failure to obtain approval for any of the above reasons may be made more likely due to the novel nature of our technology. Failure to obtain regulatory approval to market any of our product candidates would significantly harm our business, results of operations, and prospects.
53
Our clinical trials may fail to demonstrate adequately the safety and efficacy of our product candidates, which would prevent or delay regulatory approval and commercialization.
The clinical trials of our product candidates are, and the manufacturing and marketing of our products will be, subject to extensive and rigorous review and regulation by numerous government authorities in the United States and in other countries where we intend to test and market our product candidates. Before obtaining regulatory approvals for the commercial sale of any of our product candidates, we must demonstrate through lengthy, complex, and expensive preclinical testing and clinical trials that our product candidates are both safe and effective for use in each target indication. In particular, because our product candidates are subject to regulation as biological drug products, we will need to demonstrate that they are safe, pure, and potent for use in their target indications. Each product candidate must demonstrate an adequate risk versus benefit profile in its intended patient population and for its intended use. The risk/benefit profile required for product licensure will vary depending on these factors and may include not only the ability to show tumor shrinkage, but also adequate duration of response, a delay in the progression of the disease, and/or an improvement in survival. For example, response rates from the use of our product candidates may not be sufficient to obtain regulatory approval unless we can also show an adequate duration of response. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of preclinical studies and early clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials. The results of studies in one set of patients or line of treatment may not be predictive of those obtained in another. We expect there may be greater variability in results for products processed and administered on a patient-by-patient basis, as anticipated for our product candidates, than for “off-the-shelf” products, like small molecule drugs which are not personalized for each patient. There is typically an extremely high rate of attrition from the failure of product candidates proceeding through clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy profile despite having progressed through preclinical studies and initial clinical trials. Many companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or unacceptable safety issues, notwithstanding promising results in earlier trials. Most product candidates that begin clinical trials are never approved by regulatory authorities for commercialization.
In addition, even if our clinical trials are successfully completed, we cannot guarantee that the FDA or foreign regulatory authorities will interpret the results as we do, and more trials could be required before we submit our product candidates for approval. To the extent that the results of the trials are not satisfactory to the FDA or foreign regulatory authorities for support of a marketing application, we may be required to expend significant resources, which may not be available to us, to conduct additional trials in support of potential approval of our product candidates.
Our product candidates may cause undesirable side effects or have other properties that could halt their clinical development, prevent their regulatory approval, limit their commercial potential, or result in significant negative consequences.
As with most biological products, use of our product candidates could be associated with side effects or adverse events, which can vary in severity from minor reactions to death and in frequency from infrequent to prevalent. Undesirable side effects or unacceptable toxicities caused by our product candidates could cause us or regulatory authorities to interrupt, delay, or halt clinical trials.
The FDA or comparable foreign regulatory authorities could delay or deny approval of our product candidates for any or all targeted indications and negative side effects could result in a more restrictive label for any product that is approved. Side effects such as toxicity or other safety issues associated with the use of our product candidates could also require us or our collaborators to perform additional studies or halt development or sale of these product candidates.
If one or more of our product candidates receives marketing approval, and us or others later identify undesirable side effects caused by such products, including during any long-term follow-up observation period recommended or required for patients who receive treatment using our products, many potentially significant negative consequences could result, including:
54
In addition, adverse side effects caused by any therapeutics that may be similar in nature to our product candidates could delay or prevent regulatory approval of our product candidates, limit the commercial profile of an approved label for our product candidates, or result in significant negative consequences for our product candidates following marketing approval.
We believe that any of these events could prevent us from achieving or maintaining market acceptance of the affected product candidates and could substantially increase the costs of commercializing our product candidates, if approved, and significantly impact our ability to successfully commercialize our product candidates and generate revenues.
If we encounter difficulties enrolling patients in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the trial until our conclusion. We may experience difficulties in patient enrollment in our clinical trials for a variety of reasons, including:
In addition, our clinical trials will compete with other clinical trials for product candidates that are in the same therapeutic areas as our product candidates, and this competition will reduce the number and types of patients available to us, because some patients who might have opted to enroll in our trials may instead opt to enroll in a trial being conducted by one of our competitors. Because the number of qualified clinical investigators is limited, we may conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which will reduce the number of patients who are available for our clinical trials at such clinical trial sites. Moreover, because our product candidates represent a departure from more commonly used methods for cancer treatment, potential patients and their doctors may be inclined to use conventional therapies, such as chemotherapy and hematopoietic cell transplantation, rather than enroll patients in any future clinical trial.
Even if we can enroll a sufficient number of patients in our clinical trials, delays in patient enrollment may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of our product candidates.
55
Clinical trials are expensive, time-consuming, and difficult to design and implement, and our clinical trial costs may be higher than those for more conventional therapeutic technologies or drug products.
Clinical trials are expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. Because our product candidates are based on new technologies and manufactured on a patient-by-patient basis, we expect that we will require extensive research and development and have substantial manufacturing costs. In addition, costs to treat patients with relapsed/refractory cancer and to treat potential side effects that may result from our product candidates can be significant. Accordingly, our clinical trial costs are likely to be significantly higher per patient than those of more conventional therapeutic technologies or drug products.
In addition, one of our early-stage product candidates that is currently in preclinical development is for a novel class of injectable biologics. Development of the underlying technology may be affected by unanticipated technical, regulatory, manufacturing, or other problems, among other research and development issues, and the possible insufficiency of funds needed to complete development of this product candidate.
Our proposed personalized product candidates involve several complex and costly manufacturing and processing steps, the costs of which will be borne by us. Depending on the number of patients we ultimately enroll in our trials, and the number of trials we may need to conduct, our overall clinical trial costs may be higher than for more conventional treatments.
Our product candidates are biologics and the manufacture of our product candidates is complex and we may encounter difficulties in production, particularly with respect to process development or scaling-out of our manufacturing capabilities. If we or any of our third-party manufacturers encounter such difficulties, our ability to provide supply of our product candidates for clinical trials or our products for patients, if approved, could be delayed or stopped, or we may be unable to maintain a commercially viable cost structure.
Our product candidates are biologics and the process of manufacturing our products is complex, highly regulated, and subject to multiple risks. The manufacture of our product candidates involves complex processes, and, as a result of the complexities, the cost to manufacture biologics in general is generally higher than traditional small molecule chemical compounds, and the manufacturing process is less reliable and is more difficult to reproduce. Our manufacturing process will be susceptible to product loss or failure due to logistical issues. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects, and other supply disruptions. Further, as product candidates are developed through preclinical to late-stage clinical trials towards approval and commercialization, it is common that various aspects of the development program, such as manufacturing methods, are altered along the way in an effort to optimize processes and results. Such changes carry the risk that they will not achieve these intended objectives, and any of these changes could cause our product candidates to perform differently and affect the results of planned clinical trials or other future clinical trials.
In addition, the manufacturing process for any products that we may develop is subject to FDA and foreign regulatory authority approval process, and we will need to contract with manufacturers who can meet all applicable FDA and foreign regulatory authority requirements on an ongoing basis. If us or our CMOs are unable to reliably produce products to specifications acceptable to the FDA or other regulatory authorities, we may not obtain or maintain the approvals we need to commercialize such products. Even if we obtain regulatory approval for any of our product candidates, there is no assurance that either us or our CMOs will be able to manufacture the approved product to specifications acceptable to the FDA or other regulatory authorities, to produce it in sufficient quantities to meet the requirements for the potential launch of the product, or to meet potential future demand. Any of these challenges could delay completion of clinical trials, require bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of our product candidate, impair commercialization efforts, increase our cost of goods, and have an adverse effect on our business, financial condition, results of operations and growth prospects.
We rely on third parties to manufacture our clinical product supplies, and we intend to rely on third parties for at least a portion of the manufacturing process of our product candidates, if approved. Our business could be harmed if those third parties fail to provide it with sufficient quantities of product or fail to do so at acceptable quality levels or prices or fail to maintain or achieve satisfactory regulatory compliance.
We do not currently own any facility that may be used as our clinical-scale manufacturing and processing facility and currently relies on several outside vendors to manufacture supplies and process our product candidates. We have not yet caused our product candidates to be manufactured or processed on a commercial scale and may not be able to do so for any of our product candidates.
56
Although in the future we intend to develop our own manufacturing facility, we also intend to use third parties as part of our manufacturing process and may, in any event, never be successful in developing our own manufacturing facility. We anticipate reliance on a limited number of third-party manufacturers exposes us to the following risks:
Each of these risks could delay or prevent the completion of our clinical trials or the approval of any of our product candidates by the FDA, result in higher costs or adversely impact commercialization of our product candidates. In addition, we will rely on third parties to perform certain specification tests on our product candidates prior to delivery to patients. If these tests are not appropriately done and test data are not reliable, patients could be put at risk of serious harm and the FDA could place significant restrictions on us until deficiencies are remedied.
Although our agreements with our CMOs require them to perform according to certain cGMP and, if applicable, cGTP requirements such as those relating to quality control, quality assurance, and qualified personnel, we cannot control the conduct of our CMOs to implement and maintain these standards. If any of our CMOs cannot successfully manufacture material that conforms to its specifications and the regulatory requirements of the FDA, EMA, or other comparable foreign authorities, we would be prevented from obtaining regulatory approval for our drug candidates unless and until we engages a substitute CMO that can comply with such requirements, which we may not be able to do. Any such failure by any of our CMOs would significantly impact our ability to develop, obtain regulatory approval for, or market our drug candidates, if approved.
The manufacture of biological drug products is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls.
57
Manufacturers of biologic products often encounter difficulties in production, particularly in scaling up or out, validating the production process, and assuring high reliability of the manufacturing process (including the absence of contamination). These problems include logistics and shipping, difficulties with production costs and yields, quality control, including stability of the product, product testing, operator error, availability of qualified personnel, as well as compliance with strictly enforced federal, state, and foreign regulations. Furthermore, if contaminants are discovered in our supply of our product candidates or in the manufacturing facilities, such manufacturing facilities may need to be closed for an extended period to investigate and remedy the contamination. We cannot assure you that any stability failures or other issues relating to the manufacture of our product candidates will not occur in the future. Additionally, our manufacturers may experience manufacturing difficulties due to resource constraints, labor disputes, or unstable political environments. If our manufacturers were to encounter any of these difficulties, or otherwise fail to comply with their contractual obligations, our ability to provide our product candidate to patients in clinical trials would be jeopardized. Any delay or interruption in the supply of clinical trial supplies could delay the completion of clinical trials, increase the costs associated with maintaining clinical trial programs, and, depending upon the period of delay, require us to begin new clinical trials at additional expense or terminate clinical trials completely.
Our third-party manufacturers may be unable to successfully scale up manufacturing of our product candidates in sufficient quality and quantity, which would delay or prevent us from developing our product candidates and commercializing any approved product candidates.
Our manufacturing partners may be unable to successfully increase the manufacturing capacity for our product candidates in a timely or cost-effective manner, or at all, as needed for our development efforts or, if our product candidates are approved, our commercialization efforts. Quality issues may also arise during scale-up activities. If us, or any manufacturing partners, are unable to successfully scale up the manufacture of our product candidates in sufficient quality and quantity, the development, testing, and clinical trials of our product candidates may be delayed or infeasible, and regulatory approval or commercial launch of any resulting therapeutic may be delayed or not obtained, which could significantly harm our business.
Cell-based therapies rely on the availability of reagents, specialized equipment, and other specialty materials, which may not be available to us on acceptable terms or at all. For some of these reagents, equipment, and materials, we rely or may rely on sole source vendors or a limited number of vendors, which could impair our ability to manufacture and supply our products.
Manufacturing our product candidates will require many reagents, which are substances used in our manufacturing processes to bring about chemical or biological reactions, and other specialty materials and equipment, some of which are manufactured or supplied by small companies with limited resources and experience to support commercial biologics production. We currently depend on a limited number of vendors for certain materials and equipment used in the manufacture of our product candidates. Some of these suppliers may not have the capacity to support commercial products manufactured under cGMP by biopharmaceutical firms or may otherwise be ill-equipped to support our needs. We also do not have supply contracts with many of these suppliers and may not be able to obtain supply contracts with them on acceptable terms or at all. Accordingly, we may experience delays in receiving key materials and equipment to support clinical or commercial manufacturing.
For some of these reagents, equipment, and materials, we rely and may in the future rely on sole source vendors or a limited number of vendors. An inability to continue to source product from any of these suppliers, which could be due to regulatory actions or requirements affecting the supplier, adverse financial or other strategic developments experienced by a supplier, labor disputes or shortages, unexpected demands, or quality issues, could adversely affect our ability to satisfy demand for our product candidates, which could adversely and materially affect our product sales and operating results or our ability to conduct clinical trials, either of which could significantly harm our business.
As we continue to develop and scale our manufacturing process, we expect that we will need to obtain rights to and supplies of certain materials and equipment to be used as part of that process. We may not be able to obtain rights to such materials on commercially reasonable terms, or at all, and if we are unable to alter our process in a commercially viable manner to avoid the use of such materials or find a suitable substitute, we would have a material adverse effect on our business.
We rely and will rely on third parties to conduct our clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines or comply with regulatory requirements, we may not be able to obtain regulatory approval of or commercialize our product candidates.
We depend and will depend upon independent investigators and collaborators to conduct our clinical trials under agreements with universities, medical institutions, CROs, strategic partners, and others. We expect to have to negotiate budgets and contracts with CROs and trial sites, which may result in delays to our development timelines and increased costs.
58
We rely and will rely heavily on third parties over the course of our clinical trials, and as a result will have limited control over the clinical investigators and limited visibility into our day-to-day activities. Nevertheless, we are responsible for ensuring that each of our trials is conducted in accordance with the applicable protocol and legal, regulatory, and scientific standards, and our reliance on third parties does not relieve it of our regulatory responsibilities. Us and these third parties are required to comply with good clinical practices (“GCP”), which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for product candidates in clinical development. Regulatory authorities enforce these GCP through periodic inspections of trial sponsors, principal investigators, and trial sites. If we or any of these third parties fails to comply with applicable GCP regulations, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional nonclinical or clinical trials before approving our marketing applications. We cannot be certain that, upon inspection, such regulatory authorities will determine that any of our clinical trials comply with the applicable GCP regulations. In addition, our clinical trials must be conducted with biologic product produced under cGMP, and likely cGTP regulations and will require a large number of test patients. Our failure or any failure by these third parties to comply with these regulations or to recruit a sufficient number of patients may require us to repeat clinical trials, which would delay the regulatory approval process. Moreover, our business may be implicated if any of these third parties violates federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws.
Any third parties conducting our clinical trials are not and will not be our employees and, except for remedies available to us under our agreements with such third parties, we cannot control whether or not they devote sufficient time and resources to our ongoing preclinical, clinical, and nonclinical programs. These third parties may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials or other drug development activities, which could affect their performance on our behalf. If these third parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our clinical trials may be extended, delayed, or terminated and we may not be able to complete development of, obtain regulatory approval of or successfully commercialize our product candidates. As a result, our financial results and the commercial prospects for our product candidates would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
Any agreements governing our relationships with CROs or other contractors with whom we currently engages or may engage in the future may provide those outside contractors with certain rights to terminate a clinical trial under specified circumstances. If any of our relationships with these third-party CROs terminate, we may not be able to enter into arrangements with alternative CROs or do so on commercially reasonable terms. Switching or adding additional CROs involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new CRO begins work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition, and prospects.
We plan to seek orphan drug status for some or all of our product candidates, but we may be unable to obtain such designations or to maintain the benefits associated with orphan drug status, including market exclusivity, which may cause our revenue, if any, to be reduced.
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, defined as a disease or condition with a patient population of fewer than 200,000 in the United States, or a patient population greater than 200,000 in the United States when there is no reasonable expectation that the cost of developing and making available the drug or biologic in the United States will be recovered from sales in the United States for that drug or biologic. Orphan drug designation must be requested before submitting a BLA. In the United States, orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages, and user-fee waivers. After the FDA grants orphan drug designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
If a product that has orphan drug designation subsequently receives the first FDA approval for a particular active ingredient for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a BLA, to market the same biologic for the same indication for seven years, except in limited circumstances such as a showing of clinical superiority to the product with orphan drug exclusivity or if FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. As a result, even if one of our drug candidates receives orphan exclusivity, the FDA can still approve other drugs that have a different active ingredient for use in treating the same indication or disease. Furthermore, the FDA can waive orphan exclusivity if we are unable to manufacture sufficient supply of our product.
59
We plans to seek orphan drug designation for some or all of our product candidates in specific orphan indications in which there is a medically plausible basis for the use of these products, but exclusive marketing rights in the United States may be limited if we seek approval for an indication broader than the orphan designated indication and may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition. In addition, although we intend to seek orphan drug designation for other product candidates, we may never receive such designations.
The review processes of regulatory authorities are lengthy, time consuming, expensive and inherently unpredictable. If we are unable to obtain approval for our product candidates from applicable regulatory authorities, we will not be able to market and sell those product candidates in those countries or regions and our business could be substantially harmed.
The research, testing, manufacturing, labeling, approval, sale, marketing and distribution of drug products are, and will remain, subject to extensive regulation by the FDA in the United States and by the respective regulatory authorities in other countries where regulations differ. We are not permitted to market our biological product candidates in the United States until we receives the respective approval of a BLA from the FDA, or in any foreign countries until we receive the requisite approval from the respective regulatory authorities in such countries. The time required to obtain approval, if any, by the FDA, EMA and comparable foreign authorities is unpredictable, but typically takes many years following the commencement of clinical trials, if approval is obtained at all, and depends upon numerous factors, including the substantial discretion of the regulatory authorities and the type, complexity and novelty of the product candidates involved. Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional nonclinical studies or clinical trials. We have limited experience in planning and conducting the clinical trials required for marketing approvals, and we have and expect to continue to rely on third-party CROs to assist us in this process. Obtaining marketing approval requires the submission of extensive nonclinical and clinical data and supporting information to regulatory authorities for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing process, and in many cases the inspection of manufacturing, processing, and packaging facilities by the regulatory authorities. Our product candidates may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude us obtaining marketing approval or prevent or limit commercial use, or there may be deficiencies in cGMP compliance by us or by our CMOs that could result in the candidate not being approved. Moreover, we have not obtained regulatory approval for any drug candidate in any jurisdiction and it is possible that none of our existing drug candidates or any drug candidates we may seek to develop in the future will ever obtain regulatory approval.
Our biological product candidates could fail to receive, or could be delayed in receiving, regulatory approval for many reasons, including any one or more of the following:
60
Even if we were able to obtain regulatory approval in one or more jurisdictions, regulatory authorities may approve any of our product candidates for fewer or more limited indications than our requests, may not approve prices we may propose to charge for our products, may grant approval contingent on the performance of costly post-marketing clinical trials (referred to as “conditional” or “accelerated” approval depending on the jurisdiction), or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that drug candidate. Any of the foregoing circumstances could materially harm the commercial prospects for our drug candidates.
We currently have no marketing and sales organization and has no experience in marketing products. If we are unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell our product candidates, we may not be able to generate product revenue.
We currently have no sales, marketing, or commercial product distribution capabilities and have no experience in marketing products. We intend to develop an in-house marketing organization and sales force, which will require significant capital expenditures, management resources, and time. We will have to compete with other pharmaceutical and biotechnology companies to recruit, hire, train, and retain marketing and sales personnel.
If we are unable or decide not to establish internal sales, marketing and commercial distribution capabilities for any or all products we develop, it will likely pursue collaborative arrangements regarding the sales and marketing of our products. However, there can be no assurance that we will be able to establish or maintain such collaborative arrangements, or if we are able to do so, that they will have effective sales forces. Any revenue we receive will depend upon the efforts of such third parties, which may not be successful. We may have little or no control over the marketing and sales efforts of such third parties, and our revenue from product sales may be lower than if we had commercialized our product candidates itself. We also face competition in our search for third parties to assist it with the sales and marketing efforts of our product candidates.
There can be no assurance that we will be able to develop in-house sales and commercial distribution capabilities or establish or maintain relationships with third-party collaborators to successfully commercialize any product in the United States or overseas, and as a result, we may not be able to generate product revenue.
We may form or seek collaborations or strategic alliances or enter into additional licensing arrangements in the future, and we may not realize the benefits of such alliances or licensing arrangements.
We may form or seek strategic alliances, create joint ventures or collaborations, or enter into additional licensing arrangements with third parties that we believe will complement or augment our development and commercialization efforts with respect to our product candidates and any future product candidates that we may develop. Any of these relationships may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute our existing stockholders, or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for our product candidates because they may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view our product candidates as having the requisite potential to demonstrate safety and efficacy.
Further, collaborations involving our product candidates, such as our collaborations with third-party research institutions, are subject to numerous risks, which may include the following:
61
As a result, if we enter into collaboration agreements and strategic partnerships or license our products or businesses, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations and company culture, which could delay our timelines or otherwise adversely affect our business. We also cannot be certain that, following a strategic transaction or license, we will achieve the revenue or specific net income that justifies such transaction. Any delays in entering into new collaborations or strategic partnership agreements related to our product candidates could delay the development and commercialization of our product candidates in certain geographies for certain indications, which would harm our business prospects, financial condition, and results of operations.
If we engage in future acquisitions or strategic partnerships, this may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities, and subject us to other risks.
We may evaluate various acquisitions and strategic partnerships, including licensing or acquiring complementary products, intellectual property rights, technologies, or businesses. Any potential acquisition or strategic partnership may entail numerous risks, including:
62
In addition, if we undertake future acquisitions, we may issue dilutive securities, assume or incur debt obligations, incur large one-time expenses and acquire intangible assets that could result in significant future amortization expense. Moreover, we may not be able to locate suitable acquisition opportunities and this inability could impair our ability to grow or obtain access to technology or products that may be important to the development of our business.
Our internal computer systems, or those used by our third-party research institution collaborators, CROs or other contractors or consultants, may fail or suffer security breaches.
Despite the implementation of security measures, our internal computer systems and those of our future CROs and other contractors and consultants are vulnerable to damage from computer viruses and unauthorized access. Although to our knowledge we have not experienced any such material system failure or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data.
Likewise, we rely on our third-party research institution collaborators for research and development of our product candidates and other third parties for the manufacture of our product candidates and to conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidates could be delayed.
Although we take reasonable steps to help protect confidential and other sensitive information from unauthorized access or disclosure, we also could be the target of phishing attacks seeking confidential information regarding our employees. Furthermore, while we have implemented data privacy and security measures in an effort to comply with applicable laws and regulations relating to privacy and data protection, some PHI and other PII or confidential information may be transmitted to us by third parties, who may not implement adequate security and privacy measures, and it is possible that laws, rules and regulations relating to privacy, data protection, or information security may be interpreted and applied in a manner that is inconsistent with our practices or those of third parties who transmit PHI and other PII or confidential information to us.
To the extent we or these third parties are found to have violated such laws, rules or regulations or that any disruption or security breach were to result in a loss of, or damage to, our or its third-party vendors’, collaborators’ or other contractors’ or consultants’ data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability including litigation exposure, penalties and fines, we could become the subject of regulatory action or investigation, our competitive position could be harmed and the further development and commercialization of our product candidates could be delayed. Any of the above could have a material adverse effect on our business, financial condition, results of operations or prospects.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability as a result of the clinical testing of our product candidates and will face an even greater risk if we commercialize any products. For example, we may be sued if our product candidates cause or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourself against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. Even successful defense would require significant financial and management resources. Product liability claims could delay or prevent completion of our development programs. If we succeed in marketing any approved products, these claims could result in an FDA investigation of the safety and effectiveness of our products, our manufacturing processes and facilities (or the manufacturing processes and facilities of our third-party manufacturer) or our marketing programs, a recall of our products or more serious enforcement action, limitations on the approved indications for which they may be used or suspension or withdrawal of approvals. Regardless of the merits or eventual outcome, liability claims may also result in:
63
Our Risks Relating to Government Regulation
The FDA regulatory approval process is lengthy, time-consuming, and inherently unpredictable, and we may experience significant delays in the clinical development and regulatory approval, if any, of our product candidates.
The research, testing, manufacturing, labeling, approval, selling, import, export, adverse event reporting, record keeping, advertising, promotion, and distribution of drug products, including biologics, are subject to extensive regulation by the FDA and other regulatory authorities in the United States. We are not permitted to market any biological drug product in the United States until we receive a Biologics License from the FDA. We have not previously submitted a BLA to the FDA, or similar approval filings to comparable foreign authorities. However, A BLA must include extensive preclinical and clinical data and supporting information to establish that the product candidate is safe, pure, potent, and effective for each desired indication. The BLA must also include significant information regarding the chemistry, manufacturing, and controls for the product, and the manufacturing facilities must complete a successful pre-license inspection. We expect the novel nature of our product candidates to create further challenges in obtaining regulatory approval. The FDA may also require a panel of experts, referred to as an Advisory Committee, to deliberate on the adequacy of the safety and efficacy data to support licensure. The opinion of the Advisory Committee, although not binding, may have a significant impact on our ability to obtain licensure of the product candidates based on the completed clinical trials. Accordingly, the regulatory approval pathway for our product candidates may be uncertain, complex, expensive, and lengthy, and approval may not be obtained.
In addition, clinical trials can be delayed or terminated for a variety of reasons, including delays or failures related to:
64
Our third-party research institution collaborators may also experience similar difficulties in completing ongoing clinical trials and conducting future clinical trials of product candidates. Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, but a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from those in the United States, including additional preclinical studies or clinical trials as clinical studies conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval.
Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. If we fail to comply with the regulatory requirements in international markets and/or to receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our product candidates will be harmed.
Even if we receive regulatory approval of our product candidates, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our product candidates.
If our product candidates are approved, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post- marketing studies, and submission of safety, efficacy, and other post-market information, including both federal and state requirements in the United States and requirements of comparable foreign regulatory authorities.
Manufacturers and manufacturers’ facilities are required to comply with extensive FDA, and comparable foreign regulatory authority, requirements, including ensuring that quality control and manufacturing procedures conform to current Good Manufacturing Practices, or cGMP, and in certain cases Good Tissue Practices (“cGTP”), regulations. As such, us and our contract manufacturers will be subject to continual review and inspections to assess compliance with cGMP and cGTp and adherence to commitments made in any BLA, other marketing application, and previous responses to inspection observations. Accordingly, us and others with whom we work must continue to expend time, money, and effort in all areas of regulatory compliance, including manufacturing, production, and quality control.
Any regulatory approvals that we receive for our product candidates may be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including phase 4 clinical trials and surveillance to monitor the safety and efficacy of the product candidate. The FDA may also require a REMS program as a condition of approval of our product candidates, which could entail requirements for long-term patient follow-up, a medication guide, physician communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. In addition, if the FDA or a comparable foreign regulatory authority approves our product candidates, we will have to comply with requirements including submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMPs, cGTP and cGCPs for any clinical trials that we conduct post-approval.
65
Later discovery of previously unknown problems with our product candidates, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in the following among other things:
The FDA strictly regulates marketing, labeling, advertising, and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
Non-compliance with European Union requirements regarding safety monitoring or pharmacovigilance can also result in significant financial penalties. Similarly, failure to comply with the European Union’s requirements regarding the protection of personal information can also lead to significant penalties and sanctions.
The policies of the FDA and of other regulatory authorities may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability.
In addition, if we are able to obtain accelerated approval of any of our product candidates, the FDA would require us to conduct a confirmatory study to verify the predicted clinical benefit and additional safety studies. The results from the confirmatory study may not support the clinical benefit, which would result in the approval being withdrawn. While operating under accelerated approval, we will be subject to certain restrictions that we would not be subject to upon receiving regular approval.
66
Even if we obtain regulatory approval of our product candidates, the products may not gain market acceptance among physicians, patients, hospitals, cancer treatment centers, and others in the medical community. Our products may not become broadly accepted by physicians, patients, hospitals, cancer treatment centers, and others in the medical community. Several factors will influence whether our product candidates are accepted in the market, including:
Our ability to negotiate, secure and maintain third-party coverage and reimbursement for our product candidates may be affected by political, economic and regulatory developments in the United States, the European Union and other jurisdictions. Governments continue to impose cost containment measures, and third-party payors are increasingly challenging prices charged for medicines and examining their cost effectiveness, in addition to their safety and efficacy. These and other similar developments could significantly limit the degree of market acceptance of any product candidate of ours that receives marketing approval in the future.
Even if our products achieve market acceptance, we may not be able to maintain that market acceptance over time if new products or technologies are introduced that are more favorably received than our products, are more cost effective or render our products obsolete.
We are and will be subject to stringent privacy laws, cybersecurity laws, regulations, policies and contractual obligations related to privacy and security, and changes in such laws, regulations, policies or how they are interpreted or changes in related contractual obligations could adversely affect our business.
67
We are subject to data privacy and protection laws and regulations that apply to the collection, transmission, processing, storage and use of personally-identifying information including comprehensive regulatory systems in the U.S. and EU, which, among other things, impose certain requirements relating to the privacy, security and transmission of personal information. The legislative and regulatory landscape for privacy and data protection continues to evolve worldwide, and there has been an increasing focus on privacy and data protection issues with the potential to affect our business. Failure to comply with any of these laws and regulations by us or third parties to whom we contract certain types of work (like clinical trials) could result in enforcement action against us or such third parties, including fines, imprisonment of company officials and public censure, claims for damages by affected individuals, damage to our reputation and loss of goodwill, any of which could have a material adverse effect on our business, financial condition, results of operations or prospects.
There are numerous U.S. federal and state laws and regulations related to the privacy and security of personal information. In particular, regulations promulgated pursuant to the HIPAA, establish privacy and security standards that limit the use and disclosure of individually identifiable health information, or protected health information, and require the implementation of administrative, physical and technological safeguards to protect the privacy of protected health information and ensure the confidentiality, integrity and availability of electronic protected health information. Determining whether protected health information has been handled in compliance with applicable privacy standards and its contractual obligations can be complex and may be subject to changing interpretation.
If we are unable to properly protect the privacy and security of protected health information or other personal, sensitive, or confidential information in our possession, we could be found to have breached our contracts. Further, if we fail to comply with applicable privacy laws, including applicable HIPAA privacy and security standards, we could face significant administrative, civil and criminal penalties. Enforcement activity can also result in financial liability and reputational harm, and responses to such enforcement activity can consume significant internal and outside resources. In addition, state attorneys general are authorized to bring civil actions seeking either injunctions or damages in response to violations that threaten the privacy of state residents. In addition to the risks associated with enforcement activities and potential contractual liabilities, our ongoing efforts to comply with evolving laws and regulations at the federal and state level may be costly and require ongoing modifications to our policies, procedures and systems.
In the EU, we may be subject to the General Data Protection Regulation (“GDPR”) which went into effect in May 2018 and which imposes obligations on companies that operate in our industry with respect to the processing of personal data and the cross-border transfer of such data. The GDPR imposes onerous accountability obligations requiring data controllers and processors to maintain a record of their data processing and policies. If us or our partners’ or service providers’ privacy or data security measures fail to comply with the GDPR requirements, we may be subject to litigation, regulatory investigations, enforcement notices requiring we to change the way we uses personal data and/or fines of up to 20 million Euros or up to 4% of the total worldwide annual turnover of the preceding financial year, whichever is higher, as well as compensation claims by affected individuals, negative publicity, reputational harm and a potential loss of business and goodwill.
The GDPR may also impose additional compliance obligations relating to the transfer of data between us and our subsidiaries or other business partners. For example, the European Court of Justice recently invalidated the EU-U.S. Privacy Shield as a basis for transfers of personal data from the EU to the U.S. and raised questions about the continued validity of one of the primary alternatives to the EU-U.S. Privacy Shield, namely the European Commission’s Standard Contractual Clauses. Some customers or other service providers may respond to these evolving laws and regulations by asking us to make certain privacy or data-related contractual commitments that we are unable or unwilling to make. This could lead to the loss of current or prospective customers or other business relationships.
While we continue to address the implications of the recent changes to EU data privacy regulations, data privacy remains an evolving landscape at both the domestic and international level, with new regulations coming into effect and continued legal challenges, and our efforts to comply with the evolving data protection rules may be unsuccessful. It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our practices. We must devote significant resources to understanding and complying with this changing landscape. Failure to comply with laws regarding data protection would expose us to risk of enforcement actions taken by data protection authorities in the EU and elsewhere and carries with it the potential for significant penalties if we are found to be non-compliant. Similarly, failure to comply with federal and state laws in the United States regarding privacy and security of personal information could expose us to penalties under such laws. Any such failure to comply with data protection and privacy laws could result in government-imposed fines or orders requiring that we change its practices, claims for damages or other liabilities, regulatory investigations and enforcement action, litigation and significant costs for remediation, any of which could adversely affect our business.
68
Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could harm our business, financial condition, results of operations or prospects. Coverage and reimbursement may be limited or unavailable in certain market segments for our product candidates, which could make it difficult for us to sell our product candidates profitably. Successful sales of our product candidates, if approved, depend on the availability of adequate coverage and reimbursement from third-party payors. In addition, because our product candidates represent new approaches to treat cancer and other immune-related diseases, we cannot accurately estimate the potential revenue from our product candidates.
Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Adequate coverage and reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors are critical to new product acceptance.
Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which drugs and treatments they will cover and the amount of reimbursement. Coverage and reimbursement by a third-party payor may depend upon a number of factors, including the third-party payor’s determination that use of a product is:
In the United States, no uniform policy of coverage and reimbursement for products exists among third- party payors. As a result, obtaining coverage and reimbursement approval of a product from a government or other third-party payor is a time-consuming and costly process that could require us to provide to each payor supporting scientific, clinical and cost-effectiveness data for the use of our products on a payor-by-payor basis, with no assurance that coverage and adequate reimbursement will be obtained. Even if we obtain coverage for a given product, the resulting reimbursement payment rates might not be adequate for us to achieve or sustain profitability or may require co-payments that patients find unacceptably high. Additionally, third-party payors may not cover, or provide adequate reimbursement for, long-term follow-up evaluations required following the use of our products. Patients are unlikely to use our product candidates unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our product candidates. Because our product candidates have a higher cost of goods than conventional therapies, and may require long-term follow up evaluations, the risk that coverage and reimbursement rates may be inadequate for us to achieve profitability may be greater.
We intend to seek approval to market our product candidates in both the United States and in selected foreign jurisdictions. If we obtain approval in one or more foreign jurisdictions for our product candidates, we will be subject to rules and regulations in those jurisdictions. In some foreign countries, particularly those in the EU, the pricing of biologics is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after obtaining marketing approval of a product candidate. In addition, market acceptance and sales of our product candidates will depend significantly on the availability of adequate coverage and reimbursement from third-party payors for our product candidates and may be affected by existing and future health care reform measures.
Our employees, independent contractors, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of fraud, misconduct or other illegal activity by our employees, independent contractors, consultants, commercial partners and vendors. Misconduct by these parties could include intentional, reckless and negligent conduct that fails to: comply with applicable laws and regulations of the FDA and other similar foreign regulatory bodies; provide true, complete and accurate information to the FDA and other similar foreign regulatory bodies; comply with manufacturing standards we have established; comply with healthcare fraud and abuse laws in the United States and similar foreign fraudulent misconduct laws; or report financial information or data accurately or to disclose unauthorized activities to us. If we obtain FDA approval of any of our product candidates and begins commercializing those products in the United States, our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. These laws may impact, among other things, our current activities with principal investigators and research patients, as well as proposed and future sales,
69
marketing and education programs. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, structuring and commission(s), certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials, which could result in significant regulatory sanctions and cause serious harm to our reputation. It is not always possible to identify and deter misconduct by employees and other parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending itself or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
Our relationships with prescribers, purchasers, third-party payors and patients will be subject to applicable anti-kickback, fraud and abuse and other health care laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Although we do not currently have any products on the market, upon commercialization of our drug candidates, if approved, we will be subject to additional health care statutory and regulatory requirements and oversight by federal and state governments in the United States as well as foreign governments in the jurisdictions in which we conduct business. Physicians, other health care providers and third-party payors will play a primary role in the recommendation, prescription and use of any product candidates for which we obtain marketing approval. Our future arrangements with such third parties may expose us to broadly applicable fraud and abuse and other health care laws and regulations that may constrain our business or financial arrangements and relationships through which we market, sell and distribute any products for which we may obtain marketing approval. Restrictions under applicable domestic and foreign health care laws and regulations include, but are not limited to, the following:
70
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent health care reform legislation has strengthened these laws. For example, the Affordable Care Act (the “ACA”), among other things, amends the intent requirement of the federal Anti- Kickback Statute and criminal healthcare fraud statutes. As a result of such amendment, a person or entity no longer needs to have actual knowledge of these statutes or specific intent to violate them in order to have committed a violation. Moreover, the ACA provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
Efforts to ensure that our business arrangements will comply with applicable healthcare laws may involve substantial costs. It is possible that governmental and enforcement authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If any such actions are instituted against us, and we is not successful in defending ourself or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil, criminal and administrative penalties, damages, disgorgement, monetary fines, imprisonment, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations. In addition, the approval and commercialization of any of our product candidates outside the United States will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws. If any of the physicians or other health care providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from federal health care programs.
Risks Relating to Our Intellectual Property
We could be unsuccessful in obtaining or maintaining adequate patent protection for one or more of our products or product candidates.
We anticipate that we will file additional patent applications both in the United States and in other countries, as appropriate. However, we cannot predict:
71
For biological and pharmaceutical products, claims directed to compositions of matter are generally considered to be the strongest form of intellectual property protection. Such claims are not directed to any particular use of the product, and therefore encompass all uses. We cannot be certain, however, that the claims in our pending patent applications covering the composition of matter of our product candidates will be considered patentable by the United States Patent and Trademark Office (“USPTO”) or foreign patent offices, or that we issued claims will be considered valid and enforceable by U.S. or foreign courts.
Claims directed to methods of use protect the use of a product for the specified method. This type of claim does not prevent a competitor from making and marketing a product that is identical to the product for a specific use that falls outside the scope of the patented method. Moreover, even if competitors do not actively promote their product for our targeted indications, physicians may prescribe these products “off-label” for those uses that are covered by our method claims. Although off-label prescriptions may infringe or contribute to the infringement of method claims, the practice is common and such infringement is difficult to prevent or prosecute. Many of our issued claims cover methods for making our cell therapy products.
Claims directed to methods of making a product protect the process by which a product is made. This type of claim does not prevent a competitor from marketing a product that is identical to our product, if the competitor’s product is made by a process outside the scope of the patented method.
The strength of patents in the biotechnology and pharmaceutical field can be uncertain, and evaluating the scope of such patents involves complex legal and scientific analyses. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our product candidates, methods of making our product candidates, or uses thereof in the United States or in other foreign countries. Even if the patents do successfully issue, third parties may challenge the validity, enforceability, or scope thereof, which may result in such patents being narrowed, invalidated, or held unenforceable. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property or prevent others from designing their products to avoid being covered by our claims. If the breadth or strength of protection provided by the patents and patent applications we hold with respect to our product candidates is threatened, this could dissuade companies from collaborating with us to develop, and could threaten our ability to commercialize, our product candidates. Further, if we encounter delays in our clinical trials, the period of time during which we could market our product candidates under patent protection would be reduced. Because patent applications in the United States and most other countries are confidential for a period of time after filing, we cannot be certain that we are the first to file any patent application related to our product candidates.
Confidentiality agreements with employees and third parties may not prevent unauthorized disclosure of trade secrets and other proprietary information.
In addition to the protection afforded by patents, we seek to rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce, and any other elements of our product discovery and development processes that involve proprietary know-how, information, or technology that is not covered by patents. Trade secrets, however, may be difficult to protect. We seek to protect our proprietary processes, in part, by entering into confidentiality agreements with our employees, consultants, outside scientific advisors, contractors, and collaborators. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, outside scientific advisors, contractors, and collaborators might intentionally or inadvertently disclose our trade secret information to competitors. In addition, competitors may otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Furthermore, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent unauthorized material disclosure of our intellectual property to third parties, or misappropriation of our intellectual property by third parties, we will not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, operating results, and financial condition.
Third-party claims of intellectual property infringement against us or our collaborators may prevent or delay our product discovery and development efforts.
Our commercial success depends in part on us avoiding infringement of the patents and proprietary rights of third parties. There is a substantial amount of litigation involving patents and other intellectual property rights in the biotechnology and
72
pharmaceutical industries, as well as administrative proceedings for challenging patents, including interference, derivation, and reexamination proceedings before the USPTO or oppositions and other comparable proceedings in foreign jurisdictions. Recently, due to changes in U.S. law referred to as patent reform, procedures including inter parties review and post-grant review have been implemented. As stated above, this reform adds uncertainty to the possibility of challenge to our patents in the future.
Numerous U.S. and foreign issued patents and pending patent applications owned by third parties exist in the fields in which we are developing our product candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may give rise to claims of infringement of the patent rights of others.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, maintaining, and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can have a different scope and strength than do those in the United States. To date, in addition to the United States, we have filed patent applications in Australia, Brazil, Canada, China, Europe (via European Patent Office), Hong Kong, India, Israel, Japan, Russian Federation, South Korea, Mexico, and Singapore. In addition, the laws of some foreign countries, such as China, Brazil, Russia, and India, do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement against importation of infringing products is challenging or legal remedies are insufficient. These products may compete with our products and our patents or other intellectual property rights may not be effective or adequate to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, such as China, Brazil, Russia, and India, do not favor the enforcement of patents, trade secrets and other intellectual property, particularly those relating to biopharmaceutical products, which could make it difficult in those jurisdictions for us to stop the infringement or misappropriation of our patents or other intellectual property rights, or the marketing of competing products in violation of our proprietary rights. Proceedings to enforce our patent and other intellectual property rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Furthermore, such proceedings could put our patents at risk of being invalidated, held unenforceable, or interpreted narrowly, could put our patent applications at risk of not issuing, and could provoke third parties to assert claims of infringement or misappropriation against us.
We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming, and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To cease such infringement or unauthorized use, we may be required to file patent infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding or a declaratory judgment action against us, a court may decide that one or more of our patents is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceeding could put one or more of our patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put its patent applications at risk of not issuing. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business.
Interference or derivation proceedings provoked by third parties or brought by the USPTO may be necessary to determine the priority of inventions with respect to, or the correct inventorship of, our patents or patent applications or those of its licensors. An unfavorable outcome could result in a loss of our current patent rights and could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Litigation, interference, or derivation proceedings may result in a decision adverse to our interests and, even if we are successful, may result in substantial costs and distract our management and other employees.
73
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of shares of our common stock.
Issued patents covering our product candidates could be found invalid or unenforceable if challenged in court or before the USPTO or comparable foreign authority.
If us or one of our licensing partners initiate legal proceedings against a third party to enforce a patent covering one of our product candidates, the defendant could counterclaim that the patent covering our product candidate is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace, and there are numerous grounds upon which a third party can assert invalidity or unenforceability of a patent. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, inter partes review, post-grant review, and equivalent proceedings in foreign jurisdictions, such as opposition or derivation proceedings. Such proceedings could result in revocation or amendment to our patents in such a way that they no longer cover and protect our product candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity of our patents, for example, we cannot be certain that there is no invalidating prior art of which us, our patent counsel, and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Such a loss of patent protection could have a material adverse impact on our business.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves, both technological and legal complexity, and is therefore costly, time-consuming, and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. For example, in, Assoc. for Molecular Pathology v. Myriad Genetics, Inc., the U.S. Supreme Court held that certain claims to naturally-occurring substances are not patentable. Although we do not believe that any of the patents owned or licensed by us will be found invalid based on this decision, we cannot predict how future decisions by the courts, the U.S. Congress or the USPTO may impact the value of our patents.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties.
We have received confidential and proprietary information from third parties. In addition, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies. We may be subject to claims that us or our employees, consultants, or independent contractors have inadvertently or otherwise used or disclosed confidential information of these third parties or our employees’ former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial cost and be a distraction to our management and employees.
We may face competition from biosimilars, which may have a material adverse impact on the future commercial prospects of our product candidates.
Even if we is successful in achieving regulatory approval to commercialize a product candidate faster than our competitors, we may face competition from biosimilars. The Patient Protection and Affordable Care Act, which was signed into law in March 2010, included a subtitle called the Biologics Price Competition and Innovation Act of 2009 (the “BPCIA”). The BPCIA established a regulatory scheme authorizing the FDA to approve biosimilars and interchangeable biosimilars. While certain biosimilar products have been approved by the FDA for use in the United States, none of these have been cell therapy products and none have been interchangeable biosimilars. The FDA has issued several guidance documents outlining an approach to review and approval of biosimilars. Additional guidance is expected to be finalized by the FDA in the near term.
Under the BPCIA, a manufacturer may submit an application for licensure of a biologic product that is “biosimilar to” or “interchangeable with” a previously approved biological product or “reference product.” In order for the FDA to approve a biosimilar product, it must find that the product is “highly similar” to the reference product notwithstanding minor differences in clinically
74
inactive components and that there are no clinically meaningful differences between the reference product and proposed biosimilar product in terms of safety, purity, and potency. For the FDA to approve a biosimilar product as interchangeable with a reference product, the agency must find that the biosimilar product can be expected to produce the same clinical results as the reference product, and, for products administered multiple times, that the biologic and the reference biologic may be switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic.
Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date of approval of the reference product. The FDA may not approve a biosimilar product until 12 years from the date on which the reference product was approved. Even if a product is considered to be a reference product eligible for exclusivity, another company could market a competing version of that product if the FDA approves a full BLA for such product containing the sponsor’s own non-clinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity, and potency of their product. The BPCIA also created certain exclusivity periods for biosimilars approved as interchangeable products. At this juncture, it is unclear whether products deemed “interchangeable” by the FDA will, in fact, be readily substituted by pharmacies, which are governed by state pharmacy law.
If competitors are able to obtain marketing approval for biosimilars referencing our products, our products may become subject to competition from such biosimilars, with the attendant competitive pressure and consequences.
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
Although we are not currently experiencing any claims challenging the inventorship of our patents or ownership of our intellectual property, we may in the future be subject to claims that former employees, collaborators, or other third parties have an interest in our patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship. If we fail in defending any such claims, in addition to paying monetary damages, it may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Risks Relating to the Commercialization of Our Product Candidates
Our product candidates have never been manufactured on a commercial scale, and there are risks associated with scaling up manufacturing to commercial scale.
Our product candidates have never been manufactured on a commercial scale, and there are risks associated with scaling up manufacturing to commercial scale including, among others, cost overruns, potential problems with process scale-up, process reproducibility, stability issues, lot consistency and timely availability of raw materials. There is no assurance that our manufacturers will be successful in establishing a larger- scale commercial manufacturing process for our product candidates that achieves our objectives for manufacturing capacity and cost of goods. Even if we could otherwise obtain regulatory approval for any product candidate, there is no assurance that our manufacturers will be able to manufacture the approved product to specifications acceptable to the FDA or other regulatory authorities, to produce it in sufficient quantities to meet the requirements for the potential launch of the product or to meet potential future demand. If our manufacturers are unable to produce sufficient quantities of the approved product for commercialization, our commercialization efforts would be impaired, which would have an adverse effect on our business, financial condition, results of operations and growth prospects.
Biologics carry unique risks and uncertainties, which could have a negative impact on future results of operations.
The successful discovery, development, manufacturing, and sale of biologics is a lengthy, expensive, and uncertain process. There are unique risks and uncertainties with biologics. For example, access to and supply of necessary biological materials, such as cell lines, may be limited and governmental regulations restrict access to and regulate the transport and use of such materials. In addition, the development, manufacturing and sale of biologics is subject to regulations that are often more complex and extensive than the regulations applicable to other pharmaceutical products. Manufacturing biologics, especially in large quantities, is often complex and may require the use of innovative technologies. Such manufacturing also requires facilities specifically designed and validated for this purpose and sophisticated quality assurance and quality control procedures. Biologics are also frequently costly to manufacture because production inputs are derived from living animal or plant material, and some biologics cannot be made synthetically. Failure to successfully discover, develop, manufacture, and sell our biological product candidates would adversely impact our business and future results of operations.
75
Even if we are able to commercialize any of our product candidates, such products may become subject to unfavorable pricing regulations, third-party reimbursement practices or health care reform initiatives, which would harm our business.
The regulations that govern marketing approvals, pricing, coverage and reimbursement for new drug and biological products vary widely from country to country. Current and future legislation may change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product marketing approval is granted and, in some markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we may obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product, possibly for lengthy time periods, and negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more product candidates, even if our product candidates obtain marketing approval.
Our ability to commercialize any product candidates successfully also will depend in part on the extent to which coverage and reimbursement for these product candidates and related treatments will be available from government authorities, private health insurers and other organizations. In the United States, reimbursement varies from payor to payor. Reimbursement agencies in Europe may be more conservative than federal health care programs or private health plans in the United States. For example, a number of cancer drugs are generally covered and paid for in the United States, but have not been approved for reimbursement in certain European countries. A primary trend in the U.S. health care industry and elsewhere is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of payments for particular products. For example, payors may limit coverage to specific drug or biological products on an approved list, also known as a formulary, which might not include all of the FDA-approved drugs or biologics for a particular indication. Payors may require use of alternative therapies or a demonstration that a product is medically necessary for a particular patient before use of a product will be covered. Additionally, payors may seek to control utilization by imposing prior authorization requirements.
Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for products. We cannot be sure that coverage will be available for any product candidate that we commercialize and, if coverage is available, what the level of reimbursement will be. Reimbursement may impact the demand for, or the price of, any product candidate for which we obtain marketing approval. Patients are unlikely to use our products, if they are approved for marketing, unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of such products. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval.
There may be significant delays in obtaining coverage and reimbursement for newly approved drugs and biologics, and coverage may be more limited than the purposes for which the drug is approved by the FDA or comparable foreign regulatory authorities. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by federal health care programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. In the United States, third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. In the European Union, reference pricing systems and other measures may lead to cost containment and reduced prices. Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Further, there have been, and may continue to be, legislative and regulatory proposals at the U.S. federal and state levels and in foreign jurisdictions directed at broadening the availability and containing or lowering the cost of healthcare including plans announced by the Trump Administration to reform the U.S. pharmaceutical pricing system significantly through rulemaking and executive orders. In addition, existing legislation aimed at patient affordability in the United States such as the ACA may be repealed or replaced. The continuing efforts of the government, insurance companies, managed care organizations and other third-party payors to contain or reduce costs of healthcare may adversely affect our ability to set prices for our products that would allow it to achieve or sustain profitability. In addition, governments may impose price controls on any of our products that obtain marketing approval, which may adversely affect our future profitability.
76
In some foreign countries, particularly the member states of the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can be a long and expensive process after the receipt of marketing approval for a product candidate. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various EU member states and parallel distribution, or arbitrage between low-priced and high-priced member states, can further reduce prices. In some countries, we may be required to conduct additional clinical trials that compare the cost-effectiveness of our product candidates to other available therapies in order to obtain reimbursement or pricing approval. Publication of discounts by third-party payors or authorities may lead to further pressure on prices or reimbursement levels within the country of publication and other countries. If reimbursement of our products is unavailable or limited in scope or amount in a particular country, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability for sales of any of our product candidates that are approved for marketing in that country and our business could be adversely affected.
We have no experience selling, marketing or distributing products and currently have no internal marketing and sales force. If we are unable to establish effective marketing and sales capabilities or enter into agreements with third parties to market and sell our product candidates, we may not be able to effectively market and sell our product candidates, if approved, or generate product revenues.
We currently have no sales, marketing or distribution capabilities and have no experience as a company in the sale or marketing of pharmaceutical products. There can be no assurance that we will be able to market and sell our products in the United States or overseas. In order to commercialize any product candidates, we must build on a territory-by-territory basis marketing, sales, distribution, managerial and other non-technical capabilities or make arrangements with third parties to perform these services, and we may not be successful in doing so. Therefore, with respect to the commercialization of all or certain of our product candidates, we may choose to collaborate, either globally or on a territory-by-territory basis, with third parties that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems. If so, our success will depend, in part, on our ability to enter into and maintain collaborative relationships for such capabilities, such collaborators’ strategic interest in the products under development and such collaborators’ ability to successfully market and sell any such products.
If we are unable to enter into such arrangements when needed on acceptable terms or at all, we may not be able to successfully commercialize any of our product candidates that receive regulatory approval, or any such commercialization may experience delays or limitations. Further, to the extent that we depend on third parties for marketing and distribution, any revenues we receive will depend upon the efforts of such third parties, and there can be no assurance that such efforts will be successful.
To the extent that we decide not to, or is unable to, enter into collaborative arrangements with respect to the sales and marketing of our products, we may in the future need to establish an internal sales and marketing team with technical expertise and supporting distribution capabilities to commercialize our product candidates, which could be expensive, time-consuming and requiring significant attention of our executive officers to manage. Further, we may not have sufficient resources to allocate to the sales and marketing of our products.
Any failure or delay in the development of sales, marketing and distribution capabilities, through collaboration with one or more third parties or through internal efforts, would adversely impact the commercialization of any of our products that we obtain approval to market. As a result, our future product revenue will suffer and we may incur significant additional losses.
Risks Related to Our Common Stock
The market price of our common stock is expected to be volatile, and the market price of the common stock may drop.
The market price of our common stock could be subject to significant fluctuations. Some of the factors that may cause the market price of our common stock to fluctuate include:
77
Moreover, the stock markets in general have experienced substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations may also adversely affect the trading price of our common stock. In addition, a recession, depression or other sustained adverse market event resulting from rising interest rates, inflation, global geopolitical conflict, or other macroeconomic conditions could materially and adversely affect our business and the value of our common stock. In the past, following periods of volatility in the market price of a company’s securities, stockholders have often instituted class action securities litigation against such companies. Furthermore, market volatility may lead to increased shareholder activism if we experience a market valuation that activists believe is not reflective of our intrinsic value. Activist campaigns that contest or conflict with our strategic direction or seek changes in the composition of our board of directors could have an adverse effect on our operating results and financial condition.
Our Articles of Incorporation, as amended, allow for our board of directors to create new series of preferred stock without further approval by our stockholders, which could adversely affect the rights of the holders of our common stock.
Our board of directors has the authority to fix and determine the relative rights and preferences of preferred stock. Our board of directors has the authority to issue up to 5,000,000 shares of our preferred stock without further stockholder approval. As a result, our board of directors could authorize the issuance of additional series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common
78
stock and the right to the redemption of the shares, together with a premium, prior to the redemption of our common stock. In addition, our board of directors could authorize the issuance of a series of preferred stock that has greater voting power than our common stock, or that is convertible into our common stock, which could decrease the relative voting power of our common stock or result in dilution to our existing stockholders. Although we have no present intention to issue any additional shares of preferred stock or to create any additional series of preferred stock, we may issue such shares in the future.
Because we became public by means of a reverse acquisition, we may not be able to attract, or maintain, the attention of brokerage firms.
Because we became public through a “reverse acquisition”, securities analysts of brokerage firms may not provide or continue to provide coverage of us since there is little incentive to brokerage firms to recommend the purchase of our common stock. No assurance can be given that brokerage firms will want to conduct any follow-on offerings on our behalf in the future.
We do not anticipate that we will pay any cash dividends in the foreseeable future.
The current expectation is that we will retain our future earnings, if any, to fund the growth of our business as opposed to paying dividends. As a result, capital appreciation, if any, of our common stock will be our stockholders’ sole source of gain for the foreseeable future.
Risks Related to the Kineta Merger
Failure to complete the Kineta Merger could negatively impact our stock price, future business and financial results.
Our obligation to complete the Kineta Merger is subject to the satisfaction or waiver of a number of conditions set forth in the Kineta Merger Agreement. There can be no assurance that the conditions to completion of the Kineta Merger will be satisfied or waived or that the Kineta Merger will be completed. If the Kineta Merger is not completed for any reason, our ongoing businesses may be materially and adversely affected and, without realizing any of the benefits of having completed the Kineta Merger, we would be subject to a number of risks, including the following:
In addition, we could be subject to litigation related to any failure to complete the Merger or related to any proceeding to specifically enforce our obligations under the Merger Agreement.
If any of these risks materialize, they may materially and adversely affect our business, financial condition, financial results and stock prices.
The market price of our common stock will continue to fluctuate after the Kineta Merger.
Upon completion of the Kineta Merger, Kineta stockholders will become holders of our common stock. The market price of our common stock may fluctuate significantly following completion of the Kineta Merger. As a result, former current stockholders could lose some or all of the value of their investment in our common stock. In addition, any significant price or volume fluctuations in the stock market generally could have a material adverse effect on the market for, or liquidity of, the common stock received in the Kineta Merger, regardless of our actual operating performance.
79
The market price of our common stock may decline in the future as a result of the sale of shares of our common stock held by former Kineta stockholders or current stockholders.
Following their receipt of shares of our common stock as merger consideration, former Kineta stockholders may seek to sell the shares of common stock delivered to them, and, other than former directors and executive officers of Kineta who are subject to a lock-up of one-third of the shares of common stock they receive as merger consideration, the Kineta Merger Agreement contains no restriction on the ability of former Kineta stockholders to sell such shares of common stock following consummation of the Kineta Merger. Other stockholders may also seek to sell shares of our common stock held by them following, or in anticipation of, consummation of the Kineta Merger. These sales (or the perception that these sales may occur), coupled with the increase in the outstanding number of shares of our common stock, may affect the market for, and the market price of, our common stock in an adverse manner.
Satisfying closing conditions may prevent or delay completion of the Kineta Merger.
The Kineta Merger is subject to a number of conditions to closing as specified in the Kineta Merger Agreement. These closing conditions include, among others, the effectiveness of the registration statement on Form S-4 registering the issuance of shares of our common stock to Kineta stockholders in connection with the Kineta Merger, our completion of a financing resulting in net proceeds of no less than Thirty-Five Million Dollars ($35,000,000) (the “Concurrent Investment”), and the absence of any stop order or proceedings by the SEC with respect thereto, and the absence of governmental restraints or prohibitions preventing the completion of the Kineta Merger. The obligation of each of us and Kineta to complete the Kineta Merger are also conditioned on, among other things, the accuracy of certain representations and warranties of the other party on the date of the Kineta Merger Agreement and on the closing date and the compliance by such other party with certain of its covenants, in each case, subject to the materiality standards set forth in the Kineta Merger Agreement. No assurance can be given that the required stockholder approvals will be obtained or that the required conditions to closing will be satisfied (including the condition to complete a financing transaction), and, if all required consents and approvals are obtained and such conditions are satisfied, no assurance can be given as to the terms, conditions and timing of such consents and approvals. Any delay in completing the Kineta Merger could cause some or all of the benefits that we expect to achieve if the Kineta Merger is successfully completed within its expected time frame not to be realized, or to be delayed in realizing.
We need to obtain financing in connection with the Kineta Merger and cannot guarantee that we will be able to complete such financing.
Our ability to complete the contemplated Concurrent Investment will depend on, among other factors, prevailing market conditions and other factors beyond our control. We cannot provide assurance that we will be able to obtain financing on terms acceptable to it or at all, and any such failure could materially adversely affect our operations and financial condition. Our obligation to complete the Kineta Merger is conditioned upon the receipt of the Concurrent Investment. In the event such capital raise is the only condition to the closing of the Kineta Merger not otherwise satisfied, we have agreed to make a $1 million termination fee payment to Kineta if the Kineta Merger Agreement is terminated in accordance with its terms.
We will incur significant transaction and Kineta Merger-related transition costs in connection with the Kineta Merger.
We expect that we will incur significant, non-recurring costs in connection with consummating the Kineta Merger and integrating the operations of the two companies post-closing. We may incur additional costs to retain key employees. We will also incur significant fees and expenses relating to financing arrangements and legal services (including any costs that would be incurred in defending against any potential class action lawsuits and derivative lawsuits in connection with the Kineta Merger if any such proceedings are brought), accounting and other fees and costs, associated with consummating the Kineta Merger. Some of these costs are payable regardless of whether the Kineta Merger is completed. In addition, we may be required to pay a termination fee of $1,000,000 if the Kineta Merger Agreement is terminated under specified circumstances. Though we continue to assess the magnitude of these costs, additional unanticipated costs may be incurred in the Kineta Merger and the integration of our business with Kineta’s business.
We may be the target of securities class action and stockholder lawsuits which could result in substantial costs and may delay or prevent the Kineta Merger from being completed.
Securities class action lawsuits and stockholder lawsuits are often brought against public companies that have entered into merger agreements. Even if the lawsuits are without merit, defending against these claims can result in substantial costs and divert management time and resources. An adverse judgment could result in monetary damages, which could have a negative impact on our liquidity and financial condition. Additionally, if a plaintiff is successful in obtaining an injunction prohibiting completion of the Kineta Merger, then that injunction may delay or prevent the Kineta Merger from being completed, which may adversely affect our, or, if the Kineta Merger is completed but delayed, the combined company’s business, financial position and results of operations. As
80
of the date of this Annual Report, no such lawsuits have been filed in connection with the Kineta Merger and we cannot predict whether any will be filed.
General Risk Factors
Our estimates of market opportunity and forecasts of market growth may prove to be inaccurate, and even if the markets in which we compete achieve the forecasted growth, our business may not grow at similar rates, or at all.
Our market opportunity estimates and growth forecasts are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate. Our estimates and forecasts relating to size and expected growth of our target market may prove to be inaccurate. Even if the markets in which we ultimately compete meet our size estimates and growth forecasts, our business may not grow at similar rates, or at all. Our growth is subject to many factors, including our success in implementing our business strategy, which is subject to many risks and uncertainties.
Our revenue will depend, in part, upon the size of the markets in the territories for which we gain regulatory approval, the accepted price for our products, the ability to obtain coverage and reimbursement and whether we own the commercial rights for that territory. If the number of our addressable patients is not as significant as we estimate, the indication approved by regulatory authorities is narrower than we expect or the treatment population is narrowed by competition, physician choice, or treatment guidelines, we may not generate significant revenue from sales of such products, even if approved.
Our business could be adversely affected by economic downturns, inflation, increases in interest rates, natural disasters, public health crises, political crises, geopolitical events, or other macroeconomic conditions, which could have a material and adverse effect on our results of operations and financial condition.
The global economy, including credit and financial markets, has experienced extreme volatility and disruptions, including, among other things, diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, supply chain shortages, increases in inflation rates, higher interest rates, and uncertainty about economic stability. For example, the COVID-19 pandemic resulted in widespread unemployment, economic slowdown, and extreme volatility in the capital markets. The Federal Reserve has raised interest rates multiple times in response to concerns about inflation and it may raise them again.
Higher interest rates, coupled with reduced government spending and volatility in financial markets, may increase economic uncertainty and affect consumer spending. Any such volatility and disruptions may adversely affect our business or the third parties on whom we rely. If the equity and credit markets deteriorate, it may make any necessary debt or equity financing more costly, more dilutive, or more difficult to obtain in a timely manner or on favorable terms, if at all. Increased inflation rates can adversely affect us by increasing our costs, including labor and employee benefit costs.
We may in the future experience disruptions as a result of such macroeconomic conditions, including delays or difficulties in initiating or expanding clinical trials and manufacturing sufficient quantities of materials. Any one or a combination of these events could have a material and adverse effect on our results of operations and financial condition.
Item 1B. Unresolved Staff Comments.
None.
Item 1C. Cybersecurity.
Cybersecurity Risk Management and Strategy
Like many companies, we face significant and persistent cybersecurity risks. The small size of our organization and limited resources could exacerbate these risks. Our business strategy, results of operations, and financial condition have not, to date, been affected by risks from cybersecurity threats.
In the ordinary course of our business, we collect and store sensitive data, including intellectual property.
81
Governance
We aim to incorporate industry best practices for companies of our size and financial strength throughout our cybersecurity program. Our board of directors has ultimate oversight of cybersecurity risk. The Chief Executive Officer
Item 2. Properties.
The Company leases approximately 12,199 square feet of office and laboratory space in Tampa, Florida under a lease that is due to expire in March 2026. We believe that our current facilities are adequate to meet our ongoing needs, and that, if we require additional space, we will be able to obtain additional facilities on commercially reasonable terms.
Item 3. Legal Proceedings.
From time to time, we may be involved in various disputes and litigation matters that arise in the ordinary course of business. As of the date of this Annual Report, we are not party to any material legal matters or claims.
Item 4. Mine Safety Disclosure.
Not applicable.
Part II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock is traded on The Nasdaq Capital Market under the symbol “HURA.”
Stockholders
As of March 31, 2025, there were approximately 606 registered holders of record of our common stock. This number does not include “street name” or beneficial holders, whose shares are held of record by banks, brokers, financial institutions and other nominees.
Recent Sales of Unregistered Securities; Purchases of Equity Securities by the Issuer or Affiliated Purchaser
None.
Dividend Policy
We currently do not anticipate paying any cash dividends in the foreseeable future. Instead, we anticipate that all of our earnings will be used to provide working capital, to support our operations and to finance the growth and development of our business. Any future determination to declare cash dividends will be made at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements, general business conditions and other factors that our board of directors may deem relevant. In addition, if we were to enter into a credit facility in the future, we anticipate that the terms of such facility could limit or prohibit our ability to pay dividends.
Item 6. [Reserved]
82
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations together with our audited consolidated financial statements and related notes included elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth under the heading “Risk Factors” in Part I, Item 1A of this Annual Report on Form 10-K our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. See also “Forward-Looking Statements”.
In this section, we discuss our financial condition, changes in financial condition and results of our operations for the year ended December 31, 2024 compared to the year ended December 31, 2023. References to “we”, “our” and “the Company” refers to Legacy TuHURA for periods prior to the closing of the Kintara Merger, and to TuHURA Biosciences, Inc. (formerly Kintara Therapeutics, Inc.) for all other periods, as the context requires.
Overview
We are a clinical stage immuno-oncology company developing novel technologies designed to overcome primary and acquired resistance to cancer immunotherapies. Our lead product candidate, IFx2.0, is an innate immune agonist designed to overcome primary resistance to checkpoint inhibitors. We are preparing to initiate a single randomized placebo-controlled Phase 3 registration trial of IFx-2.0 administered as an adjunctive therapy to Keytruda® (pembrolizumab) in first line treatment for patients with advanced or metastatic Merkel Cell Carcinoma who are checkpoint inhibitor naïve, utilizing the FDA’s accelerated approval pathway. In addition to our innate immune agonist candidates, we are leveraging our Delta receptor technology to develop tumor microenvironment modulators in the form of first-in-class bi-specific antibody-peptide conjugates (“APCs”) and antibody-drug conjugates (“ADCs”) targeting Myeloid Derived Suppressor Cells (“MDSCs”). Our APCs and ADCs are being developed to inhibit the immune-suppressing effects of MDSCs on the tumor microenvironment to prevent T cell exhaustion and acquired resistance to checkpoint inhibitors and cellular therapies.
To date, we have devoted substantially all of our resources to organizing and staffing, business planning, raising capital, identifying and developing product candidates, enhancing our intellectual property portfolio, undertaking research, conducting preclinical studies and clinical trials, and securing manufacturing for our development programs. We do not have any products approved for sale and have not generated any revenue from product sales. We have funded our operations primarily through the private placement of capital stock and convertible notes.
We are not profitable and has incurred significant operating losses in each period since its inception, including net losses of $22.6 million for the year ended December 31, 2024, and $29.3 million for the year ended December 31, 2023 (which includes the expensing of the entire $16.2 million purchase price for the assets of TuHURA Biopharma, of which $15.0 million was paid in the form of Legacy TuHURA common stock). As of December 31, 2024, we had an accumulated deficit of $111.1 million. Our operating losses may fluctuate significantly from quarter-to-quarter and year-to-year as a result of several factors, including the timing of our preclinical studies and clinical trials and the expenditures related to other research and development activities. We expect to continue to incur operating losses. We anticipate these losses will increase substantially as it advances our product candidates through preclinical and clinical development, develops additional product candidates and seeks regulatory approvals for our product candidates. We do not expect to generate any revenues from product sales unless and until we successfully complete development and obtains regulatory approval for one or more product candidates. In addition, if we obtain marketing approval for any product candidate, we expect to incur pre-commercialization expenses and significant commercialization expenses related to marketing, sales, manufacturing and distribution. We may also incur expenses in connection with the in-licensing of additional product candidates. Furthermore, we expect to incur additional costs associated with operating as a public company, including significant legal, accounting, investor relations, compliance and other expenses that we did not previously incur as a private company.
As a result, we will need substantial additional funding to support our continuing operations and pursue our growth strategy. Until such time as we can generate significant revenue from sales of our product candidates, if ever, we expect to finance our cash needs through public or private equity offerings, debt financings, collaborations and licensing arrangements or other capital sources. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. Our failure to raise capital or enter into such other arrangements as and when needed would have a negative impact on our financial condition and could force it to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market our product candidates that we would otherwise prefer to develop and market itself.
Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when or if we will be able to achieve or maintain profitability. Even if we are able to generate product sales, we may not become profitable. If we fail to become profitable or are unable to sustain
83
profitability on a continuing basis, we may be unable to continue our operations at planned levels and be forced to reduce or terminate our operations.
As of December 31, 2024, we had cash and cash equivalents of $12.7 million. See “ — Liquidity and Capital Resources” below.
Recent Developments
Merger with Kintara Therapeutics
On October 18, 2024, we completed the transactions contemplated by the Kintara Merger Agreement. Pursuant to the Kintara Merger Agreement, Merger Sub merged with and into Legacy TuHURA with Legacy TuHURA surviving the merger and becoming Kintara’s direct, wholly-owned subsidiary. In connection with the completion of the Kintara Merger, effective at 12:01 a.m. Eastern Time on October 18, 2024, Kintara effected a 1-for-35 reverse stock split of its common stock (the “Reverse Stock Split”). Effective at 12:03 a.m. Eastern Time on October 18, 2024, the Kintara Merger was completed, and effective at 12:04 a.m. Eastern Time on October 18, 2024, Kintara changed its name to “TuHURA Biosciences, Inc.”
The Kintara Merger is being accounted for as a reverse recapitalization in accordance with U.S. GAAP, with Kintara treated as the acquired company for financial reporting purposes and TuHURA treated as the accounting acquirer. The Kintara Merger is intended to qualify for U.S. federal income tax purposes as a tax-free reorganization under the provisions of Section 368(a) of the Code.
Subject to the terms and conditions of the Kintara Merger Agreement, at the closing of the Kintara Merger, (a) each then-outstanding share of Legacy TuHURA common stock (other than shares held in treasury and excluding dissenting shares), including shares of Legacy TuHURA common stock issued upon conversion of Legacy TuHURA preferred stock and conversion of all of our convertible promissory notes issued in the Legacy TuHURA’s note financing, were converted into the right to receive a number of shares of Kintara common stock (after giving effect to the Reverse Stock Split) based on an exchange ratio of 0.1789 shares of Kintara common stock for each outstanding share of Legacy TuHURA common stock per the Kintara Merger Agreement (the “Exchange Ratio”), and (b) each then-outstanding Legacy TuHURA stock option and warrant that was not exercised immediately prior to the effective time of the Kintara Merger was assumed by Kintara with the number of underlying shares and exercise price being adjusted in accordance with the Exchange Ratio.
Also at the closing of the Kintara Merger, Kintara entered into a Contingent Value Rights Agreement with the Rights Agent (as defined in the Kintara Merger Agreement), pursuant to which holders of Kintara common stock and Kintara common stock warrants, in each case, as of the close of business on the business day immediately prior to the effective time of the Kintara Merger, received one contingent value right (a “CVR”) for each outstanding share of Kintara held by such stockholder (or, in the case of the warrants, each share of Kintara common stock for which such warrant is exercisable). Each CVR shall entitle the holder thereof to receive its portion of 1,539,918 shares of TuHURA common stock if TuHURA achieves the following milestones: (i) TuHURA enrolls a minimum of ten cutaneous metastatic breast cancer patients in a study to determine whether a dose of TuHURA’s REM-001 lower than 1.2 mg/kg elicits a treatment effect similar to that seen in prior studies of REM-001 at the 1.2 mg/kg dose and (ii) such patients enrolled in the study complete eight weeks of follow-up, in each case, on or before December 31, 2025.
Kineta Exclusivity Agreement, July 2024 Private Placement and Kineta Merger Agreement
On July 8, 2024, we issued a press release announcing that we had entered into the Exclusivity Agreement with Kineta for the potential acquisition of Kineta’s KVA12123 anti-VISTA antibody and related rights and assets associated with and derived from the asset.
KVA12123 is a rationally targeted, anti-VISTA antibody checkpoint inhibitor designed to reverse VISTA immune suppression and remodel the tumor microenvironment (TME) to overcome acquired resistance to immunotherapies.
Pursuant to the Exclusivity Agreement, among other things, Kineta granted us an exclusive right to acquire Kineta’s worldwide patents, patent rights, patent applications, product and development program assets, technical and business information, and other rights and assets associated with and derived from its development program related to KVA12123 during a specified period commencing as of July 3, 2024. Under the terms of the Exclusivity Agreement, we paid Kineta a fee in the amount of $5,000,000, with $2,500,000 paid at signing and an additional $2,500,000 paid on July 15, 2024, and we thereafter paid $300,000 in extension payments under the Exclusivity Agreement (the “Exclusivity Payments”). The Exclusivity Payments will be credited against the initial cash consideration payable to under the below-described Kineta Merger Agreement.
84
In conjunction with the Exclusivity Agreement, we sold 717,321 shares of our common stock in a private offering with a purchase price of $5,000,000 (the “July Private Placement”) to an existing (the “Investor”). In connection with the July Private Placement, the Investor is entitled to a 1.5% royalty on certain sales by us of products based on KVA12123 as set forth in the Investor’s subscription agreement. Due to the inherent uncertainties surrounding the regulatory approval of KvA12123 and future monetization, we have not allocated any of the $5,000,000 purchase price consideration to the royalty agreement.
On December 12, 2024, we announced that we had entered into the Kineta Merger Agreement for the acquisition of Kineta via a merger transaction. The Kineta Merger Agreement contemplates that, at the closing of the merger transaction, Hura Merger Sub I will (a) merge with and into Kineta, with Kineta being the surviving corporation of the First Merger, and (b) immediately following the First Merger and as part of the same overall transaction as the First Merger, the Surviving Entity will merge with and into Hura Merger Sub II, with Hura Merger Sub II being the surviving company of the Second Merger.
At the effective time of the First Merger, and subject to the terms and conditions of the Kineta Merger Agreement, each share of Kineta Common Stock issued and outstanding immediately prior to the effective time of the First Merger will be converted automatically into and will represent the right to receive, without interest, the number of shares of our common stock and cash consideration each calculated according to the terms of the Kineta Merger Agreement. The proposed Kineta Merger currently is expected to be consummated in the second quarter of 2025, subject to the satisfaction or waiver of closing conditions (including the financing condition) under the Kineta Merger Agreement.
Clinical Trial Funding Agreement
In connection with the Kineta Merger Agreement, we entered into a Clinical Trial Funding Agreement (the “CTF Agreement”) with Kineta under which we agreed to continue to fund clinical trial expenses for KVA12123 in an amount of up to $900,000, which may be increased upon mutual agreement. Pursuant to the terms of the CTF Agreement, Kineta granted a security interest to us in the assets, rights, including patents, patent rights, patent application, product and development program assets, and other rights and assets, associated with, derived from, relating to, or used in connection with KVA12123 and the KVA12123 development program and clinical trial. Any amounts loaned to Kineta under the CTF Agreement will be evidenced by a secured promissory note, bearing interest at 5% simple interest per annum, payable on the earlier of (a) following the closing of the Kineta Merger, any date on which we demand payment by written notice to Kineta or (b) if the Kineta Merger Agreement is terminated, within ten days following the date of such termination. The Kineta Merger Agreement also provides that Kineta may request the extension of up to $2,000,000 in working capital loans from us, $1,750,000 of which will be contingent on the completion of a financing transaction by us.
Special Protocol Assessment Agreement
On January 25, 2024, we entered into a Special Protocol Assessment Agreement for a single registration directed, randomized, placebo controlled Phase 3 trial for IFx-Hu2.0 as adjunctive therapy to pembrolizumab (Keytruda®) in first line treatment for patients with advanced or metastatic Merkel Cell carcinoma who are checkpoint inhibitor naive. The trial utilizes a novel design recommended by the FDA which incorporates Objective Response Rate (ORR) as the primary endpoint for accelerated approval. The trial also includes Progression Free Survival (PFS) as a key secondary endpoint which, if achieved, without demonstrating a detriment to Overall Survival, could allow conversion from accelerated approval to full approval satisfying the requirement for a post marketing trial. Before initiating this Phase 3 trial we are required to complete certain CMC activities as noted in a partial clinical hold correspondence from FDA. We have reached agreement with FDA on the requirements for lifting the partial clinical hold and believe we will meet the requirements and consequently expect to receive a complete response letter, or CRL, lifting the partial clinical hold in the second quarter of 2025. We may be in position to initiate the Phase 3 study in the second quarter of 2025 if the results of the mixing studies and potency assay qualifications are acceptable to the FDA.
Components of Our Results of Operations
Revenue
We did not generate any revenue and do not expect to generate any revenue from the sale of products in the near future.
Research and Development Expenses
To date, our research and development expenses have related primarily to the development of IFx-Hu2.0, manufacturing, clinical studies, and other early pre-clinical activities related to our portfolio. Research and development expenses are recognized as incurred, and payments made prior to the receipt of goods or services to be used in research and development are capitalized until the goods or services are received.
85
Research and development expenses include:
Clinical trial costs are a significant component of research and development expenses and include costs associated with third-party contractors. We outsource a substantial portion of our clinical trial activities, utilizing external entities such as CROs, independent clinical investigators and other third-party service providers to assist us with the execution of our clinical trials.
We plan to substantially increase our research and development expenses for the foreseeable future as we continue the development of our product candidates and seek to discover and develop new product candidates.
Due to the inherently unpredictable nature of preclinical and clinical development, we cannot determine with certainty the timing of the initiation, duration or costs of future clinical trials and preclinical studies of product candidates. Clinical and preclinical development timelines, the probability of success and the amount of development costs can differ materially from expectations. We anticipate that we will make determinations as to which product candidates and development programs to pursue and how much funding to direct to each product candidate or program on an ongoing basis in response to the results of ongoing and future preclinical studies and clinical trials, regulatory developments and our ongoing assessments as to each product candidate’s commercial potential. In addition, we cannot forecast which product candidates may be subject to future collaborations, when such arrangements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements.
Our future clinical development costs may vary significantly based on factors such as:
86
Acquired In-Process Research and Development (“IPR&D”)
Acquired in-process research and development expenses consist of existing research and development projects at the time of the acquisition. Projects that qualify as IPR&D assets represent those that have not yet reached technological feasibility and have no alternative future use. Our acquisitions of assets have included IPR&D assets that had not yet reached technological feasibility and had no alternative future use, which resulted in a write-off of these IPR&D assets as acquired in-process research and development expenses in our consolidated statement of operations.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and employee-related costs, including stock-based compensation, for personnel in our executive, finance, and other administrative functions. Other significant costs include facility related costs, legal fees relating to intellectual property and corporate matters, professional fees for accounting and consulting services and insurance costs. We anticipate that our general and administrative expenses will increase in the future to support our continued research and development activities, and, if any product candidates receive marketing approval, commercialization activities. We also anticipate increased expenses related to audit, legal, regulatory, and tax-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums and investor relations costs associated with operating as a public company.
Other Income (Expense)
Other income (expense) consists of interest income on our cash and cash equivalents, interest expense on borrowings under our convertible note agreements, and non-cash changes in the fair value of our derivative liability associated with the make-whole premium on our convertible notes. Other income (expense) also included grant income from our NIH-funded research grants completed in May 2023, employee retention tax credit for companies with employees affected during the COVID-19 pandemic, and forgiveness of a paycheck protection program loan in April 2022.
Preferred Series A Cash Dividend
Preferred Series A cash dividend represents a cash dividend payable to Valent Technologies, LLC, the holder of our Series A Preferred Stock. Effective September 30, 2014, Kintara filed a Certificate of Designation of Series A Preferred Stock with the Secretary of State of Nevada, pursuant to which, Kintara designated and thereafter issued 278,530 shares of preferred stock as Series A Preferred Stock. The shares of Series A Preferred Stock have a state value of $1.00 per share (the “Series A Stated Value”) and are not convertible into shares of our common stock. The holder of the Series A Preferred Stock is entitled to dividends at the rate of 3% of the Series A Stated Value, payable quarterly in arrears. The dividend has been recorded as a direct increase in accumulated deficit.
Warrant modification
Warrant modification represents an extension of the exercise period of common stock purchase warrants issued in connection with Legacy TuHURA Series A Preferred Stock (the “Series A Warrants”) for an additional six months, with a new expiry date of February 12, 2025. The warrant modification has been recorded as a direct increase in accumulated deficit.
Results of Operations
Comparisons for the Years Ended December 31, 2024, and December 31, 2023
87
|
|
Year ended |
|
|
|
|
||||||
|
|
December 31, |
|
|
Change |
|
||||||
|
|
2024 |
|
|
2023 |
|
|
|
|
|||
|
|
(in thousands) |
|
|
|
|
||||||
Operating expenses: |
|
|
|
|
|
|
|
|
|
|||
Research and development |
|
$ |
13,335,316 |
|
|
$ |
9,402,417 |
|
|
$ |
3,932,899 |
|
Acquired in process research and development ("IPR&D") |
|
|
- |
|
|
|
16,217,655 |
|
|
|
(16,217,655 |
) |
General and administrative |
|
|
4,314,176 |
|
|
|
4,144,648 |
|
|
|
169,528 |
|
Total operating expenses |
|
|
17,649,492 |
|
|
|
29,764,720 |
|
|
|
(12,115,228 |
) |
Loss from operations |
|
|
(17,649,492 |
) |
|
|
(29,764,720 |
) |
|
|
12,115,228 |
|
Other income (expense) |
|
|
|
|
|
|
|
|
|
|||
Employee retention tax credit |
|
|
- |
|
|
|
334,443 |
|
|
|
(334,443 |
) |
Grant income |
|
|
57,627 |
|
|
|
42,466 |
|
|
|
15,161 |
|
Interest expense |
|
|
(4,138,301 |
) |
|
|
(18,688 |
) |
|
|
(4,119,613 |
) |
Interest income |
|
|
361,632 |
|
|
|
89,673 |
|
|
|
271,959 |
|
Change in fair value of derivative liability |
|
|
(313,772 |
) |
|
|
- |
|
|
|
(313,772 |
) |
Total other income (expense) |
|
|
(4,032,814 |
) |
|
|
447,894 |
|
|
|
(4,146,265 |
) |
Net loss |
|
$ |
(21,682,306 |
) |
|
$ |
(29,316,826 |
) |
|
$ |
7,968,963 |
|
Series A Preferred cash dividend |
|
|
(2,089 |
) |
|
|
- |
|
|
|
(2,089 |
) |
Warrant modification |
|
|
(965,177 |
) |
|
|
- |
|
|
|
(965,177 |
) |
Net loss attributable to common shareholders |
|
$ |
(22,649,572 |
) |
|
$ |
(29,316,826 |
) |
|
$ |
6,667,254 |
|
Research and Development Expenses. The following table summarizes our research and development expenses by program for the periods presented.
|
|
Year ended |
|
|
|
|
||||||
|
|
December 31, |
|
|
Change |
|
||||||
|
|
2024 |
|
|
2023 |
|
|
|
|
|||
|
|
(in thousands) |
|
|
|
|
||||||
Direct program costs: |
|
|
|
|
|
|
|
|
|
|||
IFx-2.0 |
|
$ |
7,286,319 |
|
|
$ |
5,679,869 |
|
|
$ |
1,606,450 |
|
Preclinical research costs |
|
|
702,628 |
|
|
|
316,178 |
|
|
|
386,450 |
|
Indirect program costs: |
|
|
|
|
|
|
|
|
|
|||
Personnel and facilities related costs |
|
|
5,346,370 |
|
|
|
3,406,370 |
|
|
|
1,940,000 |
|
Total research and development expenses |
|
$ |
13,335,316 |
|
|
$ |
9,402,417 |
|
|
$ |
3,932,899 |
|
Research and development expenses were $13.3 million and $9.4 million for the years ended December 31, 2024, and 2023, respectively. The increase of $3.9 million related to the following.
Acquired in process research and development (“IPR&D”). On January 26, 2023, we acquired certain assets of TuHURA Biopharma, for $1.2 million in cash and 4.1 million shares of Legacy TuHURA common stock. The common shares issued to TuHURA Biopharma had an estimated fair market value of $15.0 million. We performed the “screen test” and determined that substantially all of the fair value of the gross assets acquired in the TuHURA Biopharma acquisition was concentrated in a single identifiable asset or group of similar identifiable assets. As such, the TuHURA Biopharma acquisition has been accounted for as an asset acquisition. As the underlying asset is in-process research and development, we immediately expensed the entire $16.2 million purchase price for the year ended December 31, 2023, in accordance with FASB ASC Topic 730.
General and Administrative Expenses. General and administrative expenses were $4.3 million and $4.1 million for the years ended December 31, 2024, and 2023, respectively. The increase of $0.2 million was primarily due to increases in non-cash stock
88
compensation expense and costs associated with being a public company incurred in 2024 offset by decrease in legal fees associated with the subsequently abandoned proposed merger with CohBar, Inc. which were incurred in 2023.
Employee Retention Tax Credit. The IRS provided a refundable tax credit for businesses that had employees that were affected during the COVID-19 pandemic. In October 2022, we applied for a credit under this program and in May 2023, we received notice that the credit would be $0.3 million of which $0.1 million was received in 2024.
Grant Income. Grant income was $0.1 million and less than $0.1 million for the years ended December 31, 2024 and 2023, respectively. In April 2021, we received approval from the Department of Health and Human Services for a $0.4 million grant to study cervical cancer and received reimbursements for related expenses associated with the grant. We received the final payment under this grant in May 2023. Additionally, in October 2024, we assumed the Kintara Health and Human Services grant on REM-001 and received reimbursements for related expenses associated with the grant.
Interest Expense. From December 2023 to September 2024, as part of our private placement financing under which we offered and sold convertible promissory note (the “TuHURA Notes”), we issued convertible notes totaling $31,253,000. The convertible notes included interest at 20% per annum, accretion to maturity date, and amortization of debt discount. Upon the completion of the Kintara Merger, all principal and accrued and unpaid interest and make-whole amounts under the TuHURA Notes automatically converted into shares of our common stock at a conversion price $3.80 per share of our common stock. There was no cash paid for interest in the TuHURA Notes.
Interest Income. For the years ended December 31, 2024 and 2023, respectively, interest income was earned on deposits at various banks.
Change in fair value of derivative liability. For the year ended December 31, 2024, there was a loss of $0.3 million associated with the bifurcated embedded derivative liability related to the make-whole premium on the TuHURA Notes.
Preferred Stock Series A cash dividend – The holder of our the Series A Preferred Stock received dividends payable quarterly in arrears, at an annual rate of 3% of the Series A Stated Value.
Warrant modification – For the year ended December 31, 2024, there was a $1.0 million deemed dividend due to extending the exercise period on certain of the Series A Warrants for an additional six months.
Liquidity and Capital Resources
We have incurred net losses and negative cash flows from operations since our inception and we anticipate that we will continue to incur net losses for the foreseeable future. We incurred net losses of $22.6 million and $29.3 million for the years ended December 31, 2024, and 2023, respectively, and used $14.7 million and $12.0 million of cash from our operating activities for the years ended December 31, 2024, and 2023, respectively. As of December 31, 2024, we had an accumulated deficit of $111.1 million. The $29.3 million loss for the year ended December 31, 2023, included the expensing of the entire $16.2 million purchase price for the assets of TuHURA Biopharma, of which $15.0 million was paid in the form of our common stock.
As of December 31, 2024, we had cash and cash equivalents of $12.7 million.
Sources of Liquidity
To date, we have financed our operations principally through private placements of our common and preferred stock (which, in the case of Legacy TuHURA, have all since been converted into shares of Legacy TuHURA common stock and exchanged for shares of Kintara common stock in connection with the completion of the Kintara Merger) and issuance of convertible notes that were converted into Legacy TuHURA common stock prior the Kintara Merger). Since inception, Legacy TuHURA raised approximately $41.6 million in net proceeds through the sale of its preferred stock and approximately $36.0 million in aggregate principal amount through the issuance of convertible notes.
Preferred Stock Financings by Legacy TuHURA
During the period from August 2017 through December 2020, Legacy TuHURA engaged in a series of preferred stock financings that resulted in aggregate net proceeds of $41.6 million, which preferred stock was converted into shares of Kintara common stock as a part of the Kintara Merger. As a part of such preferred stock financings, Legacy TuHURA issued warrants to purchase Legacy TuHURA common stock that were converted as a part of the Kintara Merger into warrants purchase an aggregate of approximately 8.1 million shares of our common stock.
89
Legacy TuHURA Note Financing
On April 2, 2024, we completed a private placement under which we offered and sold the TuHURA Notes to approximately 40 accredited investors during the period from December 2023 through April 2, 2024 (the “TuHURA Note Financing”). In the transaction, we received subscriptions for an aggregate principal amount of $31.3 million of TuHURA Notes, of which the entire amount was funded as of September 30, 2024.
The TuHURA Notes were general unsecured obligations of ours that had a maturity date of December 1, 2025, and that bore interest at a rate of 20% per annum, simple interest. The TuHURA Notes contained a make-whole provision under which, upon payment or conversion of the TuHURA Notes, the holders of the notes were to receive additional interest equal to the amount of interest that would have accrued through the first anniversary of the initial closing of the TuHURA Note Financing (if the notes were paid or converted prior to such first anniversary), through the 18-month anniversary of the initial closing (if the notes are paid or converted on or after the first anniversary and before the 18-month anniversary), or though the maturity date (if the notes are paid or converted after the 18-month anniversary of the initial closing).
Pursuant to the term of the TuHURA Notes, upon the completion of the Kintara Merger, all principal and accrued and unpaid interest and make-whole amounts under the TuHURA Notes automatically converted into shares of our common stock at a conversion price $3.80 per share.
In the TuHURA Note Financing, the investors that purchased at least $4.0 million in principal amount of TuHURA Notes, together with their affiliates, were issued warrants to purchase additional shares of Legacy TuHURA common stock, which warrants were converted in the Kintara Merger into warrants to purchase an aggregate of approximately 3.4 million additional shares of Kintara common stock (the “TuHURA Common Warrants”). The TuHURA Common Warrants have an exercise price of $5.70 per share of our common stock and have an expiration date of 3 years following the respective issue dates of the warrants. The TuHURA Common Warrants are exercisable at any time prior to the expiration date of the warrants, and the warrants are exercisable for cash and, at such time as there is no registration statement covering the resale of the shares issuable upon the exercise of the warrants, on a cashless basis. The TuHURA Common Warrants contain customary adjustments to the exercise price and number of underlying warrant shares by reason of stock splits, stock dividends, reverse stock split, and the like.
In connection with the TuHURA Note Financing, an aggregate of approximately 0.1 million shares of our common stock (calculated on a post-Kintara Merger basis) were issued to a placement agent for the private placement of the TuHURA Note Financing.
Private Placement of Common Stock by Legacy TuHURA
In July 2024, approximately 0.7 million shares of our common stock (calculated on a post-Kintara merger basis) were issued and sold in a private offering by Legacy TuHURA with a purchase price of $5.0 million to an existing stockholder.
Warrant Exercise Notes
On February 12, 2025, four holders (the “Makers”) of common stock purchase warrants (the “Warrants”) of the Company made and issued to the Company secured promissory notes (the “Warrant Exercise Notes”) in the aggregate principal amount of $3,011,373 as payment of the exercise price of an aggregate of 1,034,836 Warrants held by the Makers. The Makers were comprised of KP Biotech Group, LLC, CA Patel F&F Investments, LLC, Dr. Kiran C. Patel and Donald Wojnowski. Upon the exercise of the Warrants, the Company issued to the Makers an aggregate of 1,034,836 Warrant Shares, all of which are “restricted securities” within the meaning of the federal securities laws. The Warrant Exercise Notes are due and payable on May 30, 2025.
Cash Flows
The following table sets forth a summary of the net cash flow activity for the years ended December 31, 2024 and 2023, respectively:
90
|
|
Year Ended |
|
|||||
|
|
December 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
|
|
(in thousands) |
|
|||||
Net cash provided by (used in): |
|
|
|
|
|
|
||
Operating activities |
|
$ |
(14,728,138 |
) |
|
$ |
(11,950,856 |
) |
Investing activities |
|
|
(6,052,409 |
) |
|
|
(1,296,879 |
) |
Financing activities |
|
|
29,772,693 |
|
|
|
2,660,249 |
|
Net increase (decrease) in cash |
|
$ |
8,992,146 |
|
|
$ |
(10,587,486 |
) |
Operating Activities
For the year ended December 31, 2024, net cash used in operating activities was $14.7 million, which primarily consisted of a net loss of $21.7 million, a change in net operating assets and liabilities of $3.3 million, and by non-cash charges of $3.7 million. The net non-cash charges were primarily related to a $0.3 million change in fair value of derivative liability, amortization of debt discount of $1.3 million, depreciation and amortization expense of $0.1 million, and stock-based compensation of $2.0 million. The $3.3 million net change in operating assets and liabilities is primarily due to increases in accounts payable and accrued expenses of approximately $3.6 million due to timing of invoices and vendor payments, and a decrease in current and non-current assets of approximately $0.3 million.
For the year ended December 31, 2023, net cash used in operating activities was $11.9 million, which primarily consisted of a net loss of $29.3 million and a change in net operating assets and liabilities of $0.5 million, partially offset by non-cash charges of $16.9 million. The net non-cash charges were primarily related to a $16.2 million write-off of in-process research and development expense on the asset acquisition of TuHURA Biopharma, depreciation and amortization expense of $0.2 million, and stock-based compensation of $0.5 million. The $0.5 million change in net operating assets and liabilities was due to an increase in accounts payable and accrued expenses of approximately $0.3 million due to timing of invoices and vendor payments, and a decrease in current and non-current assets of approximately $0.2 million.
Investing Activities
For the year ended December 31, 2024, net cash used in investing activities was $6.1 million, which consisted of purchases of property and equipment and deposits and exclusivity payments in connection with the planned business acquisition of Kineta.
For the year ended December 31, 2023, net cash used in investing activities was $1.3 million. On January 26, 2023, TuHURA acquired certain assets of TuHURA Biopharma, for $1.2 million in cash and approximately 4.1 million common shares. The cash component of the transaction is considered an investing activity. The entire transaction was valued at $16.2 million.
Financing Activities
For the year ended December 31, 2024, net cash provided by financing activities was $29.8 million, which consisted of $27.5 million net proceeds from convertible notes issued as part of the TuHURA Note Financing, $4.7 million net proceeds from the Legacy TuHURA private placement in July 2024, and $2.0 million proceeds from stock options and warrants exercises, offset by $4.4 million in merger transaction costs and net liabilities attributable to Kintara.
For the year ended December 31, 2023, net cash provided by financing activities was $2.7 million, which primarily consisted of net proceeds from convertible notes issued as part of the TuHURA Note Financing.
Funding Requirements
We expect to incur additional costs associated with operating as a public company. In addition, we anticipate that we will need substantial additional funding in connection with our development programs and continuing operations. We believe that our existing cash and cash equivalents, together with the anticipated payment of the Warrant Exercise Notes, will be sufficient to meet our anticipated cash requirements through late into the fourth quarter of 2025. This excludes the cash needed to complete the Kineta Merger, as the Kineta Merger Agreement provides that it is a condition to the closing of the Kineta Merger that we complete a financing transaction resulting in net proceeds of no less than $35 million, and there is no assurance that we will be able to complete such a financing transaction.
91
Our forecast of the period through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially. Management based projections of operating capital requirements on our current operating plan, which includes several assumptions that may prove to be incorrect, and we may deplete our available capital resources sooner than management expects. Our future capital requirements will depend on many factors, including:
Until such time, if ever, as we can generate substantial product revenues to support our capital requirements, we expect to finance our cash needs through a combination of public or private equity offerings, debt financings, collaborations and licensing arrangements or other capital sources. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be or could be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of holders of our common stock. Debt financing and equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, or declaring dividends. If we raise funds through collaborations, or other similar arrangements with third parties, we may need to relinquish valuable rights to our product candidates, future revenue streams or research programs or may have to grant licenses on terms that may not be favorable to us and/or may reduce the value of our common stock. If we are unable to raise additional funds through equity or debt financings as and when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market our product candidates even if we would otherwise prefer to develop and market such product candidates ourselves.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our financial statements. On an ongoing basis, we evaluate our estimates and judgments, including those related to accrued expenses and stock-based compensation. We base our estimates on historical experience, known trends and events, and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Our actual results may differ from these estimates under different assumptions or conditions. While our significant accounting policies are described in more detail in Note 2 of our consolidated financial statements for the year ended December 31, 2024, contained in Item 8 in this Annual Report, we believe the following accounting policies and estimates to be most critical to the preparation of our financial statements.
Accrued Research and Development Expenses
As part of the process of preparing our financial statements, we are required to estimate our accrued expenses as of each balance sheet date. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. We make estimates of our accrued expenses as of each balance sheet date based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and adjusts, if necessary. The significant estimates in our accrued research and development
92
expenses include the costs incurred for services performed by our vendors in connection with research and development activities for which we have not yet been invoiced.
We base our expenses related to research and development activities on our estimates of the services received and efforts expended pursuant to quotes and contracts with vendors that conduct research and development on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract-to-contract and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the research and development expense. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual or prepaid expense accordingly. Advance payments for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made.
Although we do not expect our estimates to be materially different from amounts actually incurred, if our estimates of the status and timing of services performed differ from the actual status and timing of services performed, it could result in us reporting our that are too high or too low in any particular period. To date, there have been no material differences between our estimates of such expenses and the amounts actually incurred.
Stock-Based Compensation Expense
Stock-based compensation expense represents the cost of the grant date fair value of equity awards recognized over the requisite service period of the awards (usually the vesting period) on a straight-line basis. we estimate the fair value of equity awards using the Black-Scholes option pricing model and recognizes forfeitures as they occur. Estimating the fair value of equity awards as of the grant date using valuation models, such as the Black-Scholes option pricing model, is affected by assumptions regarding a number of variables, including the risk-free interest rate, the expected stock price volatility, the expected term of stock options, the expected dividend yield and the fair value of the underlying common stock on the date of grant. Changes in the assumptions can materially affect the fair value and ultimately how much stock-based compensation expense is recognized. These inputs are subjective and generally require significant analysis and judgment to develop. See Note 2 of our financial statements for information concerning certain of the specific assumptions we use in applying the Black-Scholes option pricing model to determine the estimated fair value of our stock options granted.
Common stock valuations
We are required to estimate the fair value of the common stock underlying our equity awards when performing fair value calculations. The fair value of the common stock underlying our equity awards was determined on each grant taking into account input from management and taking into account the pricing offered in our equity raises. All options to purchase shares of our common stock are intended to be granted with an exercise price per share no less than the fair value per share of our common stock underlying those options on the date of grant, based on the information known to us on the date of grant. In the absence of a public trading market for our common stock, on each grant date we develop an estimate of the fair value of our common stock in order to determine an exercise price for the option grants. Our determination of the fair value of our common stock was made by considering the prices of preferred stock sold to investors in arm’s length transactions and the rights, preferences and privileges of our preferred stock relative to those of our common stock.
In determining the fair value of shares of our underlying stock option grants prior to our reverse merger with Kintara, for the years ended December 31, 2024 and 2023, we used the market approach by reference to the closest round of equity financing, preceding the date of valuation and analysis of the trading values of publicly traded companies deemed comparable to us.
Following our reverse merger with Kintara, the fair value of our common stock will be determined based on the quoted market price of our common stock. In connection with our reverse merger with Kintara, all outstanding shares of our preferred stock were converted into shares of our common stock.
Recently Issued and Adopted Accounting Pronouncements
A description of recently issued and adopted accounting pronouncements that may potentially impact our financial position and results of operations is disclosed in Note 2 to our financial statements.
Off-Balance Sheet Arrangements
93
During the periods presented, we do not have, nor do we currently have, any off-balance sheet arrangements as defined under SEC rules.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are exposed to market risks in the ordinary course of our business. These risks primarily include interest rate risks and inflation risks. Periodically, we maintain deposits in accredited financial institutions in excess of federally insured limits. We deposit our cash in financial institutions that we believe has high credit quality and have not experienced any losses on such accounts and does not believe it is exposed to any unusual credit risk beyond the normal credit risk associated with commercial banking relationships.
Interest Rate Risk
Our cash consists of cash in readily-available checking accounts. We may also invest in short- term money market fund investments. Such interest-earning instruments carry a degree of interest rate risk; however, historical fluctuations in interest income have not been significant.
Inflation Risk
Inflation generally affects us by increasing our cost of labor and research and development contract costs. We do not believe inflation has had a material effect on our results of operations during the periods presented.
Item 8. Financial Statements and Supplementary Data.
The financial statements required to be filed pursuant to this Item 8 are appended to this Annual Report. An index of those financial statements is found in Item 15, Exhibits and Financial Statement Schedules, of this Annual Report.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating our disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Evaluation of Disclosure Controls and Procedures
Our management has evaluated, with the participation of the Chief Executive Officer and the Chief Financial Officer, the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this Annual Report. Based on this evaluation, our Chief Executive Officer and the Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of December 31, 2024.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting, as such term is defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act. Our management, under the supervision and with the participation of our principal executive officer and principal financial officer, conducted an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2024 based on the criteria set forth in “Internal Control – Integrated Framework (2013)” issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment, our management concluded that, as of December 31, 2024, our internal control over financial reporting was effective.
Attestation Report of the Registered Public Accounting Firm
94
For so long as we qualify as a non-accelerated filer, our independent registered public accounting firm is not required to issue an attestation report on our internal control over financial reporting.
Changes in Internal Control over Financial Reporting
Other than changes due to the Kintara Merger, there was no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during our most recently completed fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
95
Part III
Item 10. Directors, Executive Officers and Corporate Governance.
Executive Officers and Directors
The following table sets forth the persons who serve as our executive officers and directors, and their ages as of March 31, 2025:
Name |
|
Age |
|
Position(s) |
Executive Officers |
|
|
|
|
James Bianco, M.D. |
|
68 |
|
Chief Executive Officer and Director |
Dan Dearborn |
|
58 |
|
Chief Financial Officer |
Non-Employee Directors |
|
|
|
|
James Manuso, Ph.D., MBA |
|
76 |
|
Director and Chairman of the Board |
Alan List, M.D. |
|
70 |
|
Director |
George Ng |
|
51 |
|
Director |
Robert E. Hoffman |
|
59 |
|
Director |
Craig Tendler, M.D. |
|
66 |
|
Director |
Executive Officers
James Bianco, M.D. has served as our Chief Executive Officer and as a director since the completion of the Kintara Merger and for Legacy TuHURA since July 1, 2021. Dr. Bianco was also the founder, Chief Executive Officer and Chairman of Morphogenesis Biopharma, Inc., a biotechnology company, from its inception in November 2018 through its dissolution in January 2023, following the transfer of its assets to us. Dr. Bianco is a 30-year veteran of the biopharmaceutical industry. In 1991, Dr. Bianco founded CTI Biopharma, Inc. (“CTI”) and from 1992 to 2016 was the Chief Executive Officer of CTI. During his tenure at CTI, Dr. Bianco was responsible for strategic portfolio development and identifying, acquiring, licensing, purchasing, or acquiring through international merger and acquisition, five drug candidates, four of which have since been approved by the FDA and with three receiving accelerated or conditional regulatory approval in the U.S. and/or E.U.
Dr. Bianco earned his M.D. from the Mount Sinai Icahn School of Medicine and completed his residency and chief residency at the Mount Sinai Medical Center in New York City. He completed his fellowship in Hematology/Oncology at the University of Washington/Fred Hutchinson Cancer Research Center (FHCRC) where he was appointed Assistant Professor of Medicine, Assistant Member FHCRC and Director of the Bone Marrow Transplant Unit at a “Hutch” affiliate (SVAMC).
Dan Dearborn joined Legacy TuHURA in 2018 as its Chief Financial Officer and has served in this role for our company since the completion of the Kintara Merger. Mr. Dearborn is a CPA with over 25 years of finance experience exclusively with health care and biotechnology companies. Prior to joining our company, from 2015 to 2017, Mr. Dearborn was Chief Financial Officer at MYMD Pharmaceuticals, Inc., an emerging biotechnology firm. Mr. Dearborn is an alumnus of Loyola University in Maryland and joined Ernst & Young early in his career. He was with Pharmerica, a long-term care pharmaceutical company, for fifteen years and advanced quickly to a Director role. He then moved to BioDelivery Sciences International as Controller. During his time at BioDelivery Sciences International, the company signed two very large commercial partnership agreements and was listed on Nasdaq. Mr. Dearborn later joined Welldyne, Inc. (“Welldyne”) as its Chief Financial Officer. Welldyne is a pharmacy benefit manager that also had several related health care businesses and employed associates in Florida and Colorado. During his time with Welldyne, the company was sold to Carlyle Group, Inc., one of the largest private equity firms in the world.
Non-Employee Directors
James S. Manuso, Ph.D., MBA, has served as a director of Legacy TuHURA since November 2022 and as our director and Chairman since the completion of the Kintara Merger. Dr. Manuso has also served as Chairman and Chief Executive Officer of Talfinium Investments, Inc., an investment entity and financial consultancy, since 2014. Since 2018, Dr. Manuso has served as managing member of Laurelside LLC, a family office, which he founded. Dr. Manuso has served on the board of Ocuphire Pharma, Inc., a public company (NASDAQ:OCUP) developing Nyxol in advanced clinical trials for the treatment of multiple visual disorders, since November 2020. From 2015 until 2018, Dr. Manuso served as President, Chief Executive Officer and Vice Chairman of RespireRx Pharmaceuticals Inc. (OTC QB:RSPI), a Phase 3-ready, clinical-stage respiratory and neurological pharmaceutical company. From July 2011 until October 2013, Dr. Manuso served as Chairman and Chief Executive Officer of Astex Pharmaceuticals, Inc. (Nasdaq:ASTX) and led the sale of Astex Pharmaceuticals, Inc. to Otsuka Pharmaceutical Co., Ltd. (“Otsuka Pharmaceutical”). In
96
2013, he was a senior mergers and acquisitions advisor to Otsuka Pharmaceuticals’ executive management. Dr. Manuso has served as board chairman and chairman of the audit, governance and nominating, pricing and compensation committees of multiple companies’ boards, including Biotechnology Industry Organization, Novelos Therapeutics, Inc., Merrion Pharmaceuticals Ltd. (MERR:IEX; Dublin, Ireland), Inflazyme Pharmaceuticals, Inc. (IZP-TSE; Vancouver, Canada), Symbiontics, Inc., which he co-founded (sold to BioMarin Pharmaceutical Inc. as ZyStor, Inc.), Montigen Pharmaceuticals, Inc., Quark Pharmaceuticals, Inc., Galenica Pharmaceuticals, Inc., Supratek Pharma, Inc., EuroGen, Ltd. (London, UK), where he was chairman, and the Greater San Francisco Bay Area Leukemia & Lymphoma Society, where he also served as vice president.
Dr. Manuso holds a B.A. with honors in Economics and Chemistry from New York University, a Ph.D. in Experimental Psychology and Genetics from the New School University, and an Executive M.B.A. from Columbia Business School. Dr. Manuso is the author of numerous chapters, articles and books on topics including health care cost containment and biotechnology company management.
George Ng has served as a director of Legacy TuHURA since February 2020 and as a director of our company since the completion of the Kintara Merger. Mr. Ng has also served as a director of Calidi Biotherapeutics, Inc. (NYSE American: CLDI) since October 2019 and as its President and Chief Operating Officer since February 1, 2022, as well as a director and Chief Executive Officer of Processa Pharmaceuticals, Inc. (Nasdaq: PCSA) since August 8, 2023. In addition, Mr. Ng is currently a partner at PENG Life Science Ventures since September 2013, a director, co-founder, and chief business officer at IACTA Pharmaceuticals, Inc. since January 2020. Mr. Ng’s experience further includes serving in various executive-level positions for multiple publicly-traded and private global biotechnology and pharmaceutical firms. Mr. Ng previously served as a director of Inflammatory Response Research, Inc. from May 2019 to April 2020, as a director of Invent Medical Corp from July 2019 to January 2020, as a director of ImmuneOncia Therapeutics Inc. from June 2016 to 2019, and as a director of Virttu Biologics Limited from April 2017 to April 2019. Mr. Ng was also the Executive Vice President and Chief Administrative Officer of Sorrento Therapeutics, Inc. (Nasdaq: SRNE) from March 2015 to April 2019, the Co-Founder and President, Business of Scilex Pharmaceuticals Inc. from September 2012 to April 2019, and the Senior Vice President and General Counsel of BioDelivery Sciences International Inc. (Nasdaq: BDSI) from December 2012 to March 2015. Mr. Ng holds a JD degree from the University of Notre Dame School of Law, as well as a B.AS double degree in Biochemistry and Economics from the University of California, Davis.
Alan List, M.D. has served as a director of Legacy TuHURA since November 2022 and as director of our company since the completion of the Kintara Merger. Dr. List has also served as Chief Medical Officer of Precision BioSciences, Inc. (Nasdaq: DTIL) (“Precision BioSciences”), a clinical stage gene editing company, since April 2021 and, prior to that, had been a strategic clinical advisor to Precision BioSciences and its board since April 2020, providing advice regarding its clinical stage and pre-clinical allogeneic CAR T programs. Prior to joining Precision BioSciences, Dr. List served in various roles at the Moffitt Cancer Center, including as President and Chief Executive Officer from 2012 to December 2019, Executive Vice President, Physician in Chief from 2008 to 2012 and Chief of the Malignant Hematology Division from 2003 to 2008. Prior to joining the Moffitt Cancer Center, Dr. List held academic and clinical appointments at the University of Arizona. Dr. List is internationally recognized for his many contributions in the development of effective treatment strategies for myelodysplastic syndrome (“MDS”) and acute myeloid leukemia. His pioneering work led to the development of Revlimid (lenalidomide), a transformational treatment for patients with MDS and multiple myeloma. Dr. List is the author of numerous peer-reviewed articles and books. He previously served as the President for the Society of Hematologic Oncology as well as a member of the MDS Foundation Board of Directors. Dr. List is also an active member of the American Society of Clinical Oncology, the American Society of Hematology and the American Association for Cancer Research. He is a Charter Fellow in the National Academy of Inventors, an inductee in the Florida Inventors Hall of Fame. Dr. List received B.S. and M.S. degrees from Bucknell University and earned his M.D. from the University of Pennsylvania. He is board certified in internal medicine, hematology, and medical oncology.
Robert E. Hoffman served as a director of Kintara from April 2018 through the completion of the Kintara Merger, as Chairman of Kintara from June 2018 through the completion of the Kintara Merger, as Chief Executive Officer and President of Kintara from November 2021 through the completion of the Kintara Merger, and as interim Chief Financial Officer of Kintara from June 1, 2023 through the completion of the Kintara Merger. Mr. Hoffman was appointed to our board in connection with the completion of the Kintara Merger. He has served as a member of board of directors of ASLAN Pharmaceuticals, Inc. (Nasdaq: ASLN), a publicly-held, clinical-stage immunology focused biopharmaceutical company, since October 2018, and as a member of the board of directors of FibroGenesis, a clinical-stage regenerative medicine company, since April 2021. He has also served as a member of board of directors, on the audit committee, and on the Human Resources and compensation committee of Antibe Therapeutics Inc. (“Antibe”), a publicly-held clinical-stage biotechnology company, since November 2020, and as Chairman of Antibe’s board of directors from May 2022 to April 2024. Mr. Hoffman served as Senior Vice President and Chief Financial Officer of Heron Therapeutics, Inc., a publicly-held pharmaceutical company, from April 2017 to October 2020. From July 2015 to September 2016, Mr. Hoffman served as Chief Financial Officer of AnaptysBio, Inc., a publicly-held biotechnology company. From June 2012 to July 2015, Mr. Hoffman served as the Senior Vice President, Finance and Chief Financial Officer of Arena Pharmaceuticals, Inc. (“Arena”), a biopharmaceutical company, prior to its acquisition by Pfizer Inc. in March 2022. From August 2011 to June 2012 and
97
previously from December 2005 to March 2011, he served as Arena’s Vice President, Finance and Chief Financial Officer and in a number of various roles of increasing responsibility from 1997 to December 2005. Mr. Hoffman formerly served as a member of the board of directors of Saniona AB, a biopharmaceutical company, from September 2021 to May 2022, and as a member of the board of directors of Kura Oncology, Inc., a cancer research company, from March 2015 to August 2021. He also previously served as a member of the board of directors of CombiMatrix Corporation, a molecular diagnostics company, MabVax Therapeutics Holdings, Inc., a biopharmaceutical company, and Aravive, Inc., a clinical stage biotechnology company. Mr. Hoffman serves as a member of the steering committee of the Association of Bioscience Financial Officers. Mr. Hoffman formerly served as a director and President of the San Diego Chapter of Financial Executives International and was an advisor to the Financial Accounting Standard Board (FASB) for 10 years (2010 to 2020) advising the United States accounting rulemaking organization on emerging issues and new financial guidance. Mr. Hoffman holds a B.B.A. from St. Bonaventure University.
Craig Tendler, M.D. was appointed as a member of our board of directors on March 10, 2025. Dr. Tendler is an experienced pharmaceutical and biotech industry professional. From January 2010 through December 2024, Dr. Tendler served as the Vice President, Oncology Clinical Development, Diagnostics, and Global Medical Affairs of Johnson & Johnson Innovative Medicine Research & Development where was responsible for creating and overseeing robust development plans, including optimal integration of biomarkers and diagnostics, and comprehensive data generation activities for all products in the oncology portfolio. During his tenure at Johnson & Johnson, Dr. Tendler and his team worked in collaboration with the FDA and the European Medicines Agency to secure worldwide approvals of transformational treatment in prostate cancer, hematologic malignancies, as well as for lunch and bladder cancer. He played an instrumental role in achieving 13 FDA breakthrough designations for accelerating the early development of promising investigational medicines intended for the treatment of serious oncology conditions.
Prior to joining Johnson & Johnson Innovative Medicine, Dr. Tendler served as the Vice President of Oncology Clinical Research and Chair of the Oncology Licensing Committee at the Schering-Plough Research Institute. In addition to his pharmaceutical industry experience, Dr. Tendler has served as Co-Chair of the Friends of Cancer Research Corporate Council, member of the Bloomberg New Economy International Cancer Coalition, and member of the Admissions Committee, Mount Sinai School of Medicine. Dr. Tendler was an Assistant Professor of Pediatrics/Hematology-Oncology at the Mount Sinai School of Medicine and a NIH physician-scientist grant recipient and research fellow at the National Cancer Institute in Bethesda, Maryland. Dr. Tendler earned his undergraduate degree from Cornell University and graduated from the Mount Sinai School of Medicine, New York City, with high honors and induction into the Alpha Omega Alpha Medical Society.
Election of Officers
Our executive officers are appointed by, and serve at the discretion of, our board of directors. There are no family relationships among any of our directors or executive officers.
Board Composition
Our board of directors consists of six members. Our articles of incorporation each provides that directors are to be elected at each annual meeting of stockholders to hold office until the expiration of the term for which elected and until such director’s successor is elected and qualified or until such director’s earlier death, resignation, or removal.
Director Independence
Based on information provided by each director concerning his background, employment and affiliations, each of the directors on our board of directors, other than Dr. James Bianco and Robert E. Hoffman, qualify as independent directors, as defined under Nasdaq listing rules (the “Nasdaq listing rules”), and our board of directors consists of a majority of “independent directors,” as defined under the rules of the SEC and Nasdaq listing rules relating to director independence requirements. In addition, we are subject to the rules of the SEC and Nasdaq relating to the membership, qualifications, and operations of certain committees of our board of directors, as discussed below.
Committees of Our Board of Directors
Our board of directors has established an audit committee, a compensation committee, and a nominating and corporate governance committee, each of which operate pursuant to a charter adopted by our board of directors. Our board of directors may also establish other committees from time to time to assist our company and our board of directors.
98
Name* |
|
|
Audit Committee |
|
|
Compensation Committee |
|
|
Governance and Nominating Committee |
James Manuso, Ph.D., MBA.* |
|
|
Chairman |
|
|
Member |
|
|
|
Alan List, M.D. |
|
|
Member |
|
|
Chairman |
|
|
Member |
George Ng. |
|
|
Member |
|
|
|
|
|
Chairman |
*Chairman of our board of directors.
Audit Committee
The audit committee oversees and monitors our financial reporting process and internal control system, reviews and evaluates the audit performed by our registered independent public accountants and reports to our board of directors any substantive issues found during the audit. The audit committee is directly responsible for the appointment, compensation and oversight of the work of our registered independent public accountants. The audit committee reviews and approves all transactions with affiliated parties.
The audit committee consists of James Manuso, Ph.D., MBA (chairman), Alan List, M.D., and George Ng. Our board of directors has also reviewed the education, experience and other qualifications of each member of the audit committee and based upon such review, has determined that Dr. Manuso is an “audit committee financial expert”, as defined by the rules of the SEC.
To qualify as independent to serve on our audit committee, listing standards of Nasdaq and the applicable SEC rules require that a director not accept any consulting, advisory or other compensatory fee from us, other than for service as a director, or be an affiliated person of our company.
Compensation Committee
The compensation committee assists our board of directors in fulfilling its oversight responsibilities relating to (i) corporate governance practices and policies and (ii) compensation matters, including compensation of the directors and senior management of our company and the administration of compensation plans of our company.
The compensation committee of our company consists of Alan List, M.D. (chairman) and James Manuso, Ph.D., MBA. Each member of our compensation committee is a “non-employee” director within the meaning of Rule 16b-3 of the rules promulgated under the Exchange Act and independent within the meaning of the independent director guidelines of Nasdaq.
Nominating and Corporate Governance Committee
The nominating and corporate governance committee assesses potential candidates to fill perceived needs on our board of directors for required skills, expertise, independence and other factors. A director candidate recommended by our stockholders will be considered in the same manner as a nominee recommended by a board member, management or other sources. Stockholders wishing to recommend a candidate for nomination should contact our Secretary in writing at our Corporate Secretary, c/o TuHURA at 10500 University Center Drive, Suite 110, Tampa, FL 33612. The nominating and corporate governance committee has discretion to decide which individuals to recommend for nomination as directors.
The nominating and corporate governance committee of our company consists of George Ng (chairman) and Alan List, M.D. We believe that the composition of the nominating and corporate governance committee meets the requirements for independence under, and the functioning of such nominating and corporate governance committee complies with, any applicable requirements of the rules and regulations of Nasdaq.
Compensation Committee Interlocks and Insider Participation
Each member of the compensation committee is a “non-employee” director within the meaning of Rule 16b-3 of the rules promulgated under the Exchange Act and independent within the meaning of the independent director guidelines of Nasdaq. None of our executive officers serve as a member of our board of directors or compensation committee of any entity that has one or more executive officers who is serving on our board of directors or compensation committee.
Code of Business Conduct and Ethics for Employees, Executive Officers, and Directors
99
We adopted a Code of Ethics and Conduct that applies to all of our executive officers, financial and accounting officers, our directors, financial managers and all of our employees. Each of the members of our board of directors are committed to a high standard of corporate governance practices and, through their respective oversight roles, encourage and promote a culture of ethical business conduct. A copy of our Code of Ethics and Conduct is posted under the “Investors” tab on our website, which is located at www.tuhurabio.com.
Insider Trading Policy
Our board of directors has
Policy Relating to Recovery of Erroneously Awarded Compensation (Clawback Policy)
We have instituted a clawback policy in accordance with the Nasdaq’s final rules implementing the incentive-based compensation recovery provisions of the Dodd-Frank Wall Street Reform and Consumer Protection Act, effective October 2, 2023 to support a culture of focused, diligent and responsible management that discourages conduct detrimental to our growth. The policy applies to each person who serves as an executive officer of our company, as defined in Rule 10D-1(d) under the Exchange Act, which include our named executive officers (each, a “covered employee”). In the event of a qualifying financial restatement, a covered employee will be required to forfeit erroneously awarded incentive compensation to the Company to the extent required under applicable law.
Communications with the Board
Any securityholder or any other interested party who desires to communicate with our board of directors, our non-management directors or any specified individual director, may do so by directing such correspondence to the attention of the Secretary, TuHURA Biosciences, Inc., 10500 University Center Dr., Suite 110, Tampa, Florida 33612. The Secretary will forward the communication to the appropriate director or directors as appropriate.
Item 11. Executive Compensation.
On October 18, 2024, we completed the Kintara Merger with Legacy TuHURA. At the effective time of the Kintara Merger, our management was replaced with the management of Legacy TuHURA. Unless otherwise indicated, the disclosures in this section regarding our common stock or securities convertible into common stock for periods or as of a date that precedes the closing of the Kintara Merger have been adjusted to give effect to the Exchange Ratio and Reverse Stock Split.
This discussion may contain forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt in the future could vary significantly from our historical practices and currently planned programs summarized in this discussion
Executive Compensation Overview
Our named executive officers (“NEOs”) for the year ended December 31, 2024, which consist of each person who served as our principal executive officer and the next two most highly-compensated executive officers who served during the year ended December 31, 2024, are as follows:
100
2024 Summary Compensation Table
The following table summarizes the compensation earned by, awarded to or paid to the NEOs for the years ended December 31, 2024 and 2023:
Name and Principal Position |
|
|
Fiscal |
|
|
Salary ($) |
|
|
|
Bonus(1) |
|
|
|
Stock |
|
|
|
Option |
|
|
|
All Other |
|
|
|
|
Total ($) |
|
||||||
Dr. James Bianco |
|
|
2024 |
|
|
|
463,734 |
|
|
|
|
- |
|
|
|
|
- |
|
|
|
|
4,900,000 |
|
|
|
|
80,000 |
|
|
|
$ |
5,443,734 |
|
|
President and Chief Executive Officer |
|
|
2023 |
|
|
|
439,834 |
|
|
|
|
579,668 |
|
|
|
|
- |
|
|
|
|
192,000 |
|
|
|
|
83 |
|
|
|
$ |
|
1,211,585 |
|
Dan Dearborn |
|
|
2024 |
|
|
|
339,101 |
|
|
|
|
- |
|
|
|
|
- |
|
|
|
|
2,200,000 |
|
|
|
|
- |
|
|
|
$ |
2,539,101 |
|
|
Chief Financial Officer |
|
|
2023 |
|
|
|
320,833 |
|
|
|
|
254,326 |
|
|
|
|
- |
|
|
|
|
42,240 |
|
|
|
|
108 |
|
|
|
$ |
|
635,775 |
|
Dennis Yamashita(4) |
|
|
2024 |
|
|
|
320,833 |
|
|
|
|
- |
|
|
|
|
- |
|
|
|
|
588,000 |
|
|
|
|
- |
|
|
|
$ |
|
908,833 |
|
Former Chief Scientific Officer |
|
|
2023 |
|
|
|
- |
|
|
|
|
- |
|
|
|
|
- |
|
|
|
|
- |
|
|
|
|
- |
|
|
|
$ |
|
- |
|
Robert Hoffman(5) |
|
|
2024 |
|
|
|
717,544 |
|
|
|
|
397,669 |
|
|
|
|
- |
|
|
|
|
- |
|
|
|
1,326,016(6) |
|
|
|
$ |
2,441,229 |
|
||
Former President, Chief Executive Officer |
|
|
2023 |
|
|
|
589,600 |
|
|
|
|
178,380 |
|
|
|
|
255,923 |
|
|
|
|
160,460 |
|
|
|
|
- |
|
|
|
$ |
|
1,184,363 |
|
Narrative Disclosure to Summary Compensation Table
Employment Agreements
Dr. James Bianco. On March 29, 2024, we entered into a second amended and restated employment agreement with Dr. Bianco under which Dr. Bianco serves as our President and Chief Executive Officer for an initial term of two years, unless earlier terminated. Dr. Bianco’s employment agreement provides that he will serve as the President and Chief Executive Officer of our company. Upon the expiration of the initial two-year term, the term of Dr. Bianco’s employment agreement will automatically extend, upon the same terms and conditions, for additional periods of one year, unless, either party gives 90 days’ prior notice of its intention not to extend the term. Dr. Bianco’s annual base salary is $463,734, to be reviewed periodically by our board of directors or any compensation committee thereof. Dr. Bianco is also eligible for consideration to receive an annual incentive bonus of up to 125% of his base salary and a discretionary bonus. The amount of any incentive bonus is to be established annually based on objectives determined by our board of directors or any compensation committee thereof, and the timing and amount of any discretionary bonus is to be determined at the sole discretion of our board of directors or any compensation committee thereof. Dr. Bianco must remain employed on the date any bonus is to be paid to receive such bonus. Dr. Bianco’s employment agreement also provides that we will pay for a $2,000,000 term life insurance policy for the benefit of Dr. Bianco’s designated beneficiaries. Dr. Bianco’s employment
101
agreement provides that if Dr. Bianco’s employment is terminated for any reason, Dr. Bianco shall receive any base salary that had accrued but not been paid, payment of accrued and unused vacation time, and any reimbursement due to him pursuant to his employment agreement (“Accrued Obligations”). Additionally, if Dr. Bianco is terminated without cause, including by notice of non-extension of his employment agreement, or he resigns for good reason, as such terms are defined in his employment agreement, and he executes a release of claims in the form prescribed by us within 30 days of the termination, (A) we are obligated to pay to Dr. Bianco (i) his Accrued Obligations, (ii) two years of his base salary plus an amount equal to the average of his two prior years’ bonuses, paid in one lump sum within 30 days of the separation, and (iii) reimbursement for monthly premiums to continue health insurance for two years or until other health insurance is obtained by Dr. Bianco and (B) any unvested portion of any outstanding options or unvested shares of our common stock granted to Dr. Bianco will immediately vest and become exercisable and will remain exercisable for a period of seven years following the date of his separation. If Dr. Bianco’s termination occurs upon the same circumstances, except that it occurs immediately prior to, upon, or within two years following a Change of Control (as defined in Dr. Bianco’s employment agreement), Dr. Bianco’s bonus payment will instead be an amount equal to the greater of the average of the two prior years’ bonuses or 50% of his base salary.
Dan Dearborn. On March 29, 2024, we entered into a second amended and restated employment agreement with Mr. Dearborn under which Mr. Dearborn serves as our Chief Financial Officer for an initial term of two years, unless earlier terminated. Mr. Dearborn’s employment agreement also provides that he will serve as the Chief Financial Officer of our company. Upon the expiration of the initial two-year term, the term of Mr. Dearborn’s employment agreement will automatically extend, upon the same terms and conditions, for additional periods of one year, unless, either party gives 90 days’ prior notice of its intention not to extend the term. Mr. Dearborn’s annual base salary is $339,101, to be reviewed periodically by our board of directors or any compensation committee thereof. Mr. Dearborn is also eligible for consideration to receive an annual incentive bonus up to 100% of his base salary and a discretionary bonus. The amount of any incentive bonus is to be established annually based on objectives determined by our board of directors or any compensation committee thereof, and the timing and amount of any discretionary bonus is to be determined at the sole discretion of our board of directors or any compensation committee thereof. Mr. Dearborn must remain employed on the date any bonus is to be paid to receive such bonus. Mr. Dearborn’s employment agreement provides that if Mr. Dearborn’s employment is terminated for any reason, Mr. Dearborn shall receive his Accrued Obligations. Additionally, if Mr. Dearborn is terminated without cause, including by notice of non-extension of his employment agreement, or he resigns for good reason, as such terms are defined in his employment agreement, and he executes a release of claims in the form prescribed by us within 30 days of the termination, (A) we are obligated to pay to Mr. Dearborn (i) his Accrued Obligations, (ii) one year of his base salary plus an amount equal to the average of his two prior years’ bonuses, paid in one lump sum within 30 days of the separation, and (iii) reimbursement for monthly premiums to continue health insurance for one year or until other health insurance is obtained by Mr. Dearborn and (B) any unvested portion of any outstanding options or unvested shares of our common stock granted to Mr. Dearborn will immediately vest and become exercisable and will remain exercisable for a period of seven years following the date of his separation. If Mr. Dearborn’s termination occurs upon the same circumstances, except that it occurs immediately prior to, upon, or within two years following a Change of Control (as defined in Mr. Dearborn’s employment agreement), Mr. Dearborn’s bonus payment will instead be an amount equal to the greater of the average of the two prior years’ bonuses or 50% of his base salary.
102
Dr. Dennis Yamashita. On December 19, 2023, we entered into an employment agreement with Mr. Yamashita under which Mr. Yamashita served as our Chief Scientific Officer for an initial term of two years, unless earlier terminated. Upon the expiration of the initial two-year term, the term of Mr. Yamashita’s employment agreement will automatically extend, upon the same terms and conditions, for additional periods of one year, unless, either party gives 90 days’ prior notice of its intention not to extend the term. Mr. Yamashita’s annual base salary is $350,000, to be reviewed periodically by our board of directors or any compensation committee thereof. Mr. Yamashita also received options to purchase 196,791 shares of our common stock. Such options expire ten years from the date of the grant and vest over three years. Mr. Yamashita is also eligible for consideration to receive an annual incentive bonus up to 68% of his base salary and a discretionary bonus. The amount of any incentive bonus is to be established annually based on objectives determined by our board of directors or any compensation committee thereof, and the timing and amount of any discretionary bonus is to be determined at the sole discretion of our board of directors or any compensation committee thereof. Mr. Yamashita must remain employed on the date any bonus is to be paid to receive such bonus. Mr. Yamashita’s employment agreement provides that if Mr. Yamashita employment is terminated for any reason, Mr. Dearborn shall receive his Accrued Obligations. Additionally, if Mr. Yamashita is terminated without cause, including by notice of non-extension of his employment agreement, or he resigns for good reason, as such terms are defined in his employment agreement, and he executes a release of claims in the form prescribed by us within 30 days of the termination, (A) we are obligated to pay to Mr. Yamashita (i) his Accrued Obligations, (ii) one year of his base salary plus an amount equal to the average of his two prior years’ bonuses, paid in one lump sum within 30 days of the separation, and (iii) reimbursement for monthly premiums to continue health insurance for one year or until other health insurance is obtained by Mr. Yamashita and (B) any unvested portion of any outstanding options or unvested shares of our common stock granted to Mr. Yamashita will immediately vest and become exercisable and will remain exercisable for a period of seven years following the date of his separation. If Mr. Yamashita’s termination occurs upon the same circumstances, except that it occurs immediately prior to, upon, or within two years following a Change of Control (as defined in Mr. Yamashita’s employment agreement), Mr. Yamashita’s bonus payment will instead be an amount equal to the greater of the average of the two prior years’ bonuses or 50% of his base salary.
Base Salaries
The base salaries for Dr. Bianco, Mr. Dearborn and Dr. Yamashita for fiscal 2024 were established in connection with their employment agreements. The table below sets forth the base salary as of December 31, 2024, for each NEO.
Name |
|
|
Base Salary (as of |
|
|
|
Dr. James Bianco |
|
$ |
|
463,764 |
|
|
Dan Dearborn |
|
$ |
|
339,101 |
|
|
Robert Hoffman |
|
$ |
|
594,600 |
|
(1) |
Dr. Dennis Yamashita |
|
$ |
|
- |
|
(2) |
Annual Bonuses
Each NEO is eligible to receive an annual incentive bonus based on objectives determined by our board of directors or any compensation committee thereof.
A target annual bonus, as a percentage of base salary, is established for each NEO, as set forth in the table below. Following review of individual performance during fiscal 2024, our board of directors (or the compensation committee, as applicable) determined that it was appropriate to award the following annual bonuses for fiscal 2024.
Name |
|
Target Bonus (% |
|
|
2024 Annual |
Dr. James Bianco |
|
100% |
|
$ |
463,764 |
Dan Dearborn |
|
50% |
|
$ |
169,551 |
Dennis Yamashita |
|
68% |
|
$ |
- |
Robert Hoffman |
|
67% |
|
$ |
397,669 |
103
Equity Awards
We have historically provided long-term incentive compensation to the NEOs through grants of stock options to purchase shares of our common stock under the TuHURA Amended and Restated 2019 Equity Incentive Plan (the “2019 Plan”) and the TuHURA 2024 Equity Incentive Plan (the “2024 Plan”).
Retirement Plans
TuHURA maintains a 401(k) plan for employees, although it does not currently make matching contributions to such plan. Except for the 401(k) plan, we have not had and currently do not have a pension or other retirement plan or a nonqualified deferred compensation plan.
Other Compensation
All NEOs are eligible to participate in our employee benefit plans, including our medical, dental, vision, life and disability insurance plans, in each case on the same basis as all other employees, provided that we pay all premiums for the medical, dental, and vision plans for our executive officers. For the NEOs, we pay for and on behalf of each NEO life insurance premiums. We generally do not provide perquisites or personal benefits to our NEOs, except in limited circumstances.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth certain information regarding outstanding stock options held by the named executive officers as of December 31, 2024.
Name |
|
Number of |
|
|
|
Number of |
|
|
|
Option |
|
|
Option |
|||
Dr. James Bianco |
|
|
178,901 |
|
|
|
|
- |
|
|
|
$ |
3.69 |
|
|
7/1/2031 |
President and Chief Executive Officer |
|
|
23,853 |
|
|
|
|
47,707 |
|
(1) |
|
$ |
3.69 |
|
|
4/7/2033 |
|
|
|
191,281 |
|
(3) |
|
|
|
|
|
$ |
4.14 |
|
|
2/28/2034 |
|
|
|
|
1,065,990 |
|
(4) |
|
|
|
|
|
$ |
4.94 |
|
|
11/12/2034 |
|
Dan Dearborn |
|
|
33,991 |
|
|
|
|
- |
|
|
|
$ |
2.42 |
|
|
1/17/2026 |
Chief Financial Officer |
|
|
68,439 |
|
|
|
|
- |
|
|
|
$ |
2.42 |
|
|
12/20/2026 |
|
|
|
155,046 |
|
|
|
|
77,525 |
|
(2) |
|
$ |
3.69 |
|
|
11/15/2032 |
|
|
|
5,248 |
|
|
|
|
10,496 |
|
(1) |
|
$ |
3.69 |
|
|
4/7/2033 |
|
|
|
66,659 |
|
(3) |
|
|
|
|
|
$ |
4.14 |
|
|
2/28/2034 |
|
|
|
|
489,848 |
|
(4) |
|
|
|
|
|
$ |
4.94 |
|
|
11/12/2034 |
|
Dennis Yamashita |
|
|
|
|
|
|
196,791 |
|
|
|
$ |
4.14 |
|
|
2/28/2034 |
|
Chief Scientific Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Robert Hoffman |
|
|
2 |
|
|
|
|
- |
|
|
|
$ |
18,550.00 |
|
|
4/13/2028 |
Former President, Chief Executive Officer and Interim |
|
|
2 |
|
|
|
|
- |
|
|
|
$ |
10,673.25 |
|
|
11/8/2028 |
|
|
|
20 |
|
|
|
|
- |
|
|
|
$ |
1,067.50 |
|
|
9/5/2029 |
|
|
|
22 |
|
|
|
|
- |
|
|
|
$ |
1,067.50 |
|
|
9/5/2029 |
|
|
|
68 |
|
|
|
|
- |
|
|
|
$ |
2,975.00 |
|
|
9/15/2030 |
|
|
|
57 |
|
|
|
|
- |
|
|
|
$ |
2,170.00 |
|
|
9/22/2031 |
|
|
|
2,010 |
|
|
|
|
- |
|
|
|
$ |
1,680.00 |
|
|
11/8/2031 |
|
|
|
686 |
|
|
|
|
- |
|
|
|
$ |
307.48 |
|
|
8/1/2032 |
|
|
|
661 |
|
|
|
|
- |
|
|
|
$ |
162.93 |
|
|
8/30/2033 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
104
Potential Payments Upon Termination or Change in Control
Under the employment agreements with the NEOs, in the event the NEO is terminated by us other than for “Cause” or by the NEO for “Good Reason,” the NEO will be eligible for the following severance benefits if he executes a release of claims in the form prescribed by us within 30 days of the termination: (A) payment of (i) employee’s Accrued Obligations, (ii) two years of base salary plus an amount equal to the greater of the average of such employee’s two prior years’ bonuses or 50% of such employee’s then-base salary, paid in one lump sum within 30 days of the separation, and (iii) reimbursement for monthly premiums to continue health insurance for two years or until other health insurance is obtained by such employee and (B) any unvested portion of any outstanding options or unvested shares of our common stock granted to such employee will immediately vest and become exercisable and will remain exercisable for a period of seven years following the date of such employee’s separation. If the termination of the NEO occurs upon the same circumstances, except that it occurs immediately prior to, upon, or within two years following a Change of Control (as defined in the NEO’s employment agreements), the NEO’s bonus payment will instead be an amount equal to the greater of the average of the two prior years’ bonuses or 50% of his base salary. The Kintara Merger was not deemed a Change of Control for purposes of each NEO employment agreement, and the Kineta Merger will not be deemed a Change of Control for purposes of each NEO employment agreement.
For purposes of the employment agreements and the outstanding stock options: “Cause” is defined as (i) gross negligence or willful misconduct in the performance of employee’s duties to our company after written notice to employee and the failure to cure same within ten business days after receipt of written notice; (ii) refusal or failure to act in accordance with any lawful specific direction or order of our board of directors after written notice to employee of such refusal or failure and failure to cure the same within ten days after receipt of written notice; (iii) commission of any act of fraud with respect to us; (iv) employee’s material breach of any written agreement or material policy of ours after written notice to employee of such breach and failure to cure, if curable, the same within ten business days after receipt of written notice; and (v) employee’s conviction of, or plea of nolo contendre to, a crime which adversely affects our business or reputation, in each case as determined by our board of directors; (vi) employee’s willful unauthorized disclosure of Confidential Information (as defined in our confidential disclosure policy); (vii) continued or excessive absences or tardiness, after an official warning has been issued and failure to cure (not including authorized leaves of absence, FMLA leave, or absences that are a result of an accommodation under ADA).
Summary Description of Our Equity Plans
TuHURA (f/k/a Morphogenesis, Inc.) 2019 Amended and Restated Equity Incentive Plan
The TuHURA (f/k/a Morphogenesis, Inc.) 2019 Equity Incentive Plan (the “2019 Plan”) was approved by the Legacy TuHURA board of directors and its stockholders in January 2019. The 2019 Plan, which amended and restated the TuHURA 2016 Stock Option Plan, provided for the issuance of up to 20,000,000 shares of Legacy TuHURA common stock, which includes the number of outstanding awards made pursuant to the TuHURA 2016 Stock Option Plan. The 2019 Plan allowed for awards of incentive stock options to TuHURA’s employees, nonqualified stock options to TuHURA’s directors, restricted stock, restricted stock units, and other stock-based awards. In connection with the closing of the Kintara Merger, TuHURA (f/ka Kintara Therapeutics, Inc.) assumed all outstanding awards under the 2019 Plan. No further grants will be made under the 2019 Plan.
2024 Equity Incentive Plan
In connection with the Kintara Merger, the TuHURA Board of Directors approved the 2024 Equity Incentive Plan (the “2024 Plan”) on August 7, 2024, which was subsequently approved at a special meeting of our stockholders held on October 4, 2024. The 2024 Plan replaced the Kintara Therapeutics, Inc. 2017 Omnibus Equity Incentive Plan, as amended (the “Legacy Kintara Plan”), of which no further grants will be made under. The following is a summary of certain terms and conditions of the 2024 Plan.
Administration. Our board of directors or the compensation committee of the board of directors, or any successor committee with similar authority that our board of directors may appoint, (the “Committee”) will administer the 2024 Plan (the “Administrator”). The 2024 Plan authorizes the Administrator to interpret the provisions of the 2024 Plan and award agreements; prescribe, amend and rescind rules and regulations relating to the 2024 Plan; correct any defect, supply any omission, or reconcile any inconsistency in the
105
2024 Plan, any award or any agreement covering an award; and make all other determinations necessary or advisable for the administration of the 2024 Plan, in each case in its sole discretion.
Eligibility. The Administrator may designate any of the following as a participant from time to time, to the extent of the Administrator’s authority: any officer or other employee of our company or our affiliates; any individual who we or one of our affiliates has engaged to become an officer or employee; any consultant or advisor who provides services to our company or our affiliates; or any director, including a non-employee director.
Types of Awards. The 2024 Plan permits the grant of stock options (including incentive stock options), stock appreciation rights, performance shares, performance units, restricted stock, restricted stock units, cash incentives and other types of awards authorized under the 2024 Plan. These award types are described in further detail below.
Stock Subject to the 2024 Plan. The 2024 Plan provides that 11,000,000 shares of our common stock are reserved for issuance under the 2024 Plan, all of which may be issued pursuant to the exercise of incentive stock options. The aggregate number of shares of common stock reserved for issuance under the 2024 Plan increases annually on the first day of each fiscal year after the consummation of the Kintara Merger, commencing on the first day of our fiscal year 2025 and with a final increase on the first day of the 2034 fiscal year, by a number of shares of common stock (“Evergreen Shares”) equal to the lesser of: (i) 5.0% of the outstanding shares of all classes of our common stock as of the last day of the immediately preceding fiscal year or (ii) such other number of shares (which may be zero) as our board of directors may determine. Evergreen shares may not be issued pursuant to the exercise of incentive stock options. The number of shares reserved under the 2024 Plan will be depleted on the date of the grant of an award by the maximum number of shares, if any, with respect to which such award is granted. An award that may be settled solely in cash shall not cause any depletion of the 2024 Plan’s share reserve at the time such award is granted. In general, if an award granted under the 2024 Plan lapses, expires, terminates or is canceled without the issuance of shares under the award, if it is determined during or at the conclusion of the term of an award that all or some portion of the shares under the award will not be issuable on the basis that the conditions for such issuance will not be satisfied, if shares are forfeited under an award or if shares are issued under any award and we reacquire them pursuant to rights reserved upon the issuance of the shares, then such shares will again be available for issuance under the 2024 Plan, except that shares reacquired pursuant to reserved rights may not be issued pursuant to incentive stock options. Shares of common stock not issued or delivered as a result of the net settlement of an outstanding option or stock appreciation right, shares tendered or withheld in payment of the exercise price of an option, shares tendered or withheld to satisfy tax withholding obligations and shares purchased by us using proceeds from option exercises may not be re-credited to the reserve.
Director Award Limit. The maximum number of shares that may be subject to awards granted during a single fiscal year to any individual non-employee director, subject to appropriate adjustments in accordance with the 2024 Plan, may not exceed the number of shares that has a grant date fair value of, when added to any cash compensation received by such non-employee director, $1,000,000, except that such limit will be $2,000,000 for the first fiscal year that the non-employee director serves on the board.
Options. The Administrator will generally determine all terms and conditions of each option. However, the grant date may not be any day prior to the date that the Administrator approves the grant, the exercise price may not be less than the fair market value of the shares subject to the option as determined on the date of grant (110% of the fair market value in the case of an incentive stock option granted to a 10% stockholder) and the option must terminate no later than ten years after the date of grant (five years in the case of an incentive stock option granted to a 10% stockholder). If a participant disposes of shares issued pursuant to the exercise of an incentive stock option under the circumstances described in Code Section 421(b) (relating to certain disqualifying dispositions), that participant must notify us of such disposition within 10 days. To the extent previously approved by the Administrator (in an award agreement or in administrative rules), and subject to such procedures as the Administrator may specify, the payment of the exercise price of options may be made by payment in cash or previously owned shares, through a broker-dealer assisted sell-to-cover transaction, by withholding shares otherwise deliverable upon exercise, or a combination of the foregoing. Except to the extent otherwise set forth in an award agreement, a participant will have no rights as a holder of common stock as a result of the grant of an option until the option is exercised, the exercise price and applicable withholding taxes are paid and the shares subject to the option are issued thereunder.
Stock Appreciation Rights. The Administrator will generally determine all terms and conditions of each stock appreciation right. A stock appreciation right is the right of a participant to receive cash in an amount, and/ or common stock with a fair market value, equal to the appreciation of the fair market value of a share of common stock during a specified period of time. However, the grant date may not be any day prior to the date that the Administrator approves the grant, the grant price may not be less than the fair market value of the shares subject to the stock appreciation right as determined on the date of grant and the stock appreciation right must terminate no later than ten years after the date of grant.
Performance and Stock Awards. The Administrator will generally determine all terms and conditions of each award of shares, restricted stock, restricted stock units, performance shares or performance units. Restricted stock means shares of common
106
stock that are subject to a risk of forfeiture, restrictions on transfer or both a risk of forfeiture and restrictions on transfer. A restricted stock unit means the right to receive a payment equal to the fair market value of one share of common stock. Performance shares means the right to receive shares of common stock, including restricted stock, to the extent performance goals are achieved (or other requirements are met). A performance unit means the right to receive a cash payment or shares valued in relation to a unit that has a designated dollar value or the value of which is equal to the fair market value of one or more shares of common stock, to the extent performance goals are achieved (or other requirements are met). The Administrator will determine the length of the vesting and/or performance period.
Any participant who holds restricted stock has the right to vote their shares, unless the Administrator provides otherwise. Any participant who holds other types of awards does not have any rights as a stockholder of our company, unless the Administrator provides otherwise.
Cash Incentive Awards. The Administrator has the authority to grant cash incentive awards. A cash incentive award is the right to receive a cash payment to the extent performance goals are achieved. The Administrator will determine all of the terms and conditions of each cash incentive award, including the performance goals, the performance period, the potential amount payable and the timing of payment.
Other Stock-Based Awards. The Administrator may grant a participant shares of unrestricted stock as a replacement for other compensation to which the participant is entitled, such as in payment of director fees, in lieu of cash compensation, in exchange for cancellation of compensation right or as a bonus.
Transferability of Awards. Awards under the 2024 Plan may not be sold, transferred for value, pledged, assigned, or otherwise alienated or hypothecated, other than by will or the laws of descent and distribution, unless and to the extent the Administrator allows a participant to: (1) designate in writing a beneficiary to exercise the award or receive payment under the award after the participant’s death; (2) transfer an award to the former spouse of the participant as required by a domestic relations order incident to a divorce; or (3) transfer an award (provided the participant may not receive consideration for such transfer), provided that in each case, the assignee cannot further transfer the award. Any permitted transfer shall be subject to compliance with applicable securities laws.
Adjustments.
Under the terms of the 2024 Plan, if any of the following occurs:
Repricing Prohibited. Neither the Administrator nor any other person may, without stockholder approval: (1) amend the terms of outstanding stock options or stock appreciation rights to reduce the exercise price of such outstanding stock options or stock
107
appreciation rights; (2) cancel outstanding stock options or stock appreciation rights in exchange for stock options or stock appreciation rights with an exercise price that is less than the exercise price of the original stock options or stock appreciation rights; or (3) cancel outstanding stock options or stock appreciation rights with an exercise price above the current share price in exchange for cash or other securities.
Backdating Prohibited. The Administrator may not grant a stock option or stock appreciation right with a grant date that is effective prior to the date the Administrator takes action to approve such award.
Term of Plan. Unless our board of directors terminates the 2024 Plan on an earlier date, the 2024 Plan will terminate, and no further awards can be granted thereunder, after the 10th anniversary of the latest date on which the 2024 Plan, or any amendment thereto or restatement thereof, has been approved by our stockholders.
Termination and Amendment of the 2024 Plan. The Administrator may amend or terminate the 2024 Plan at any time, except that (1) our board of directors must approve any amendment that it is required to approve by reason of applicable law or prior action of our board, (2) stockholders must approve any amendments if such approval is required by any applicable law or the listing requirements of any principal securities exchange on which our shares are then traded, and (3) stockholders must approve any amendments that would diminish the backdating or repricing restrictions contained in the 2024 Plan.
Amendment, Modification, Cancellation and Disgorgement of Awards. Subject to exceptions specified in the 2024 Plan, the Administrator may amend or cancel an award granted under the 2024 Plan at any time, or waive any restrictions or conditions applicable to any award or the exercise of the award. In addition, the Administrator will have full power and authority to terminate or cause a participant to forfeit an award, and require the participant to disgorge any gains attributable to an award, if the participant engages in any action constituting, as determined by the Administrator in its discretion, cause for termination or a breach of a material policy, any award agreement or any other agreement concerning noncompetition, nonsolicitation, confidentiality, trade secrets, intellectual property, nondisparagement or similar obligations. All awards, and any shares issued or cash paid pursuant to an award, are also subject to any applicable recoupment or clawback policy adopted by us or any recoupment or similar requirement contained in applicable law, regulation or the listing requirements of the exchange or system on which our stock is principally traded.
Policies and Practices Related to the Timing of Grants of Certain Equity Awards
Non-Employee Director Compensation
The following table presents the total compensation for each person who served as a non-employee member of our board of directors (including the non-employee directors of Kintara prior to the consummation of the Kintara Merger) and received compensation for such service during the fiscal year ended December 31, 2024. During the fiscal year ended December 31, 2024, Mr. Toth and Mmes Johnson and Favorito served as a member of our board of directors of Kintara until their resignation effective in connection with the closing of the Kintara Merger on October 18, 2024. Messrs Manuso, List and Ng were appointed as members of our board of directors on October 18, 2024 in connection with the consummation of the Kintara Merger. Mr. Hoffman was a director on our board of directors prior to the completion of the Kintara Merger, and was appointed as a member of our board of directors to serve following the Kintara Merger. Dr. James Bianco, our Chief Executive Officer, did not receive any compensation for his service as a member of our board of directors for the fiscal year ended December 31, 2024. Dr. Bianco’s compensation for service as an employee for fiscal year 2024 is presented in the section titled “Executive Compensation—Summary Compensation Table” above.
108
Name |
|
Year |
|
Fees Earned |
|
|
Option |
|
|
Total ($) |
|
|||
James Manuso, Ph.D(1) |
|
2024 |
|
$ |
14,750 |
|
|
$ |
- |
|
|
$ |
14,750 |
|
Alan List, M.D.(2) |
|
2024 |
|
$ |
13,875 |
|
|
$ |
- |
|
|
$ |
13,875 |
|
George Ng.(3) |
|
2024 |
|
$ |
14,125 |
|
|
$ |
- |
|
|
$ |
14,125 |
|
Robert Hoffman(4) |
|
2024 |
|
$ |
11,408 |
|
|
$ |
- |
|
|
$ |
11,408 |
|
Robert J. Toth, Jr.(5) |
|
2024 |
|
$ |
49,101 |
|
|
$ |
- |
|
|
$ |
49,101 |
|
Laura Johnson (6) |
|
2024 |
|
$ |
48,302 |
|
|
$ |
- |
|
|
$ |
48,302 |
|
Tamara A. Favorito(7) |
|
2024 |
|
$ |
51,097 |
|
|
$ |
- |
|
|
$ |
51,097 |
|
On November 26, 2024, our board of directors approved of a Non-Employee Director Compensation Program (the “Non-Employee Director Compensation Program”), effective beginning January 1, 2025.
Each of our non-employee directors as of December 31, 2024 held the following outstanding options:
Name(1) |
|
Shares Subject to Outstanding Options |
James Manuso, Ph.D |
|
27,106 |
Alan List, M.D. |
|
27,106 |
George Ng |
|
62,891 |
Item 12. Security Ownership of Certain Beneficial Owners and Management Related Stockholder Matters.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table provides certain information as of December 31, 2024, with respect to all of our equity compensation plans in effect on that date:
109
|
|
Number of |
|
|
Weighted-average |
|
|
Number of |
|
||||
Plan category |
|
(a) |
|
|
(b) |
|
|
(c) |
|
||||
Equity compensation plans approved by security holders |
|
|
6,403,932 |
|
|
$ |
|
5.11 |
|
|
|
4,596,068 |
|
Equity compensation plans not approved by security holders |
|
|
- |
|
|
$ |
|
- |
|
|
|
- |
|
Total |
|
|
6,403,932 |
|
|
$ |
|
5.11 |
|
|
|
4,596,068 |
|
110
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
To our knowledge, the following table sets forth certain information regarding the beneficial ownership of our common stock as of March 31, 2025 (except as indicated below) by:
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Except as noted by a footnote, and subject to community property laws where applicable, we believe based on the information provided to us that the persons and entities named in the table below have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them.
The table lists applicable percentage ownership based on 42,680,397 shares of common stock outstanding as of the Beneficial Ownership Date. The number of shares beneficially owned includes shares of common stock that each person has the right to acquire within 60 days of the Beneficial Ownership Date, including upon the exercise of stock options, warrants and the settlement of restricted stock units. These stock options, warrants and restricted stock units shall be deemed to be outstanding for the purpose of computing the percentage of outstanding shares of common stock owned by such person but shall not be deemed to be outstanding for the purpose of computing the percentage of outstanding shares of common stock owned by any other person.
This table is based upon information supplied by our officers, directors as of the record date and the principal stockholders and Schedules 13D and 13G filed with the SEC (the dates of such filings are indicated in the footnotes).
Name of Beneficial Owner(1) |
|
Common Stock Beneficially Owned |
|
|
% |
|
||
Directors and Named Executive Officers |
|
|
|
|
|
|
||
James Bianco(2) |
|
|
2,792,575 |
|
|
|
6.3 |
% |
Dan Dearborn(3) |
|
|
295,440 |
|
|
* |
|
|
George Ng(4) |
|
|
62,891 |
|
|
* |
|
|
Alan List(5) |
|
|
27,106 |
|
|
* |
|
|
James Manuso(6) |
|
|
27,106 |
|
|
* |
|
|
Robert E. Hoffman(7) |
|
|
5,354 |
|
|
* |
|
|
Craig Tendler(8) |
|
|
- |
|
|
* |
|
|
All Officers and Directors as a group (8 Total)(9) |
|
|
3,210,472 |
|
|
|
7.4 |
% |
Greater than 5% Stockholders |
|
|
|
|
|
|
||
Vijay Patel(10) |
|
|
12,364,430 |
|
|
|
25.7 |
% |
CA Patel F&F Investments, LLC (11) |
|
|
2,572,582 |
|
|
|
5.8 |
% |
KP Biotech Group, LLC(12) |
|
|
2,572,582 |
|
|
|
5.8 |
% |
Samir Patel(13) |
|
|
2,466,377 |
|
|
|
5.7 |
% |
Charles Theofilos, M.D. (14) |
|
|
2,506,321 |
|
|
|
5.7 |
% |
|
|
|
|
|
|
|
||
*Represents beneficial ownership of less than 1%.
111
112
Item 13. Certain Relationships and Related Transactions, and Director Independence.
In addition to the compensation arrangements, including employment, termination of employment and change in control arrangements, with our directors and executive officers, including those discussed in the section titled “Executive Compensation,” in this Annual Report, the following is a description of each transaction involving our company since January 1, 2024 and each currently proposed transaction in which:
Warrant Exercise Promissory Notes
On February 12, 2025, KP Biotech Group, LLC and CA Patel F&F Investments, LLC (the “Makers”), holders of more than 5% of our capital stock and holder of certain common stock purchase warrants (the “Common Stock Warrants”), made and issued to us secured promissory notes (the “Warrant Exercise Promissory Notes”) in the aggregate principal amount of $1,301,445.12 per note as payment of the exercise price of a 447,232 Warrants held by each Maker. We issued to each Maker 447,232 shares of our common stock in connection with the Warrant Exercise Promissory Notes.
The Warrant Exercise Promissory Notes bear interest at a rate of 12% per annum, simple interest, and all interest and principal under the Warrant Exercise Promissory Notes are due and payable on or before May 30, 2025 (the “Maturity Date”). The Warrant Exercise Promissory Notes are secured by the shares of our common stock issuable upon the exercise of the Common Stock Warrants (the “Warrant Shares”). In the event that a Warrant Exercise Promissory Note is not paid in full by the Maturity Date, the interest rate on the Warrant Exercise Promissory Notes increases to 18% per annum. The Warrant Exercise Promissory Notes provide for customary events of default (each as defined in the Warrant Exercise Promissory Notes, an “Event of Default”), including, among other things, the event of nonpayment of principal, interest, fees or other amounts, failure to perform or observe covenants within a specified cure period, and the occurrence of a bankruptcy, insolvency or similar event affecting the respective Maker. The Warrant Exercise Promissory Notes shall become due and immediately payable upon an Event of Default, unless otherwise waived by us. Upon an Event of Default, we may foreclose on the Warrant Shares and/or proceed against the Maker.
Consulting Agreements
On July 1, 2021, in connection with Dr. Bianco becoming CEO, Dr. Michael Lawman and Dr. Patricia Lawman ceased to be employees and officers of Legacy TuHURA and an entity (the “Consultant”) that they own became a consultant and entered into a consulting agreement, as amended by that certain amendment dated February 14, 2022, with Legacy TuHURA for a period beginning July 1, 2021 through December 31, 2023, unless earlier terminated. Dr. Patricia Lawman and Dr. Michael Lawman served as directors of Legacy TuHURA during its fiscal years ended December 31, 2022 and 2023. Through this consulting agreement, Legacy TuHURA paid to the Consultant an annual fee of $533,000 and Dr. Michael Lawman and Dr. Patricia Lawman provided services as consultants to Legacy TuHURA. During the term of the consulting agreement, the Consultant was reimbursed for all reasonable and necessary business expenses that Consultant incurred while performing the services, including reimbursement related to continued coverage under the Consolidated Omnibus Budget Reconciliation Act. Legacy TuHURA reimbursed a total of $21,747 and $14,720 for COBRA costs in 2023 and 2022, respectively. Additionally, Consultant was granted stock options in the same amount and on the same terms as executive officers of Legacy TuHURA were granted stock options during the term of the agreement. In April 2023, Dr. Michael Lawman was granted options to purchase 42,936 shares of Legacy TuHURA common stock (as adjusted by the exchange ratio in the Kintara Merger) and Dr. Patricia Lawman was granted options to purchase 52,418 shares of Legacy TuHURA common stock (as adjusted by the exchange ratio in the Kintara Merger). This consulting agreement expired on December 31, 2023 pursuant to its contractual term. On November 12, 2024, each of Dr. Patricia Lawman and Dr. Michael Lawman were granted 138,325 options to purchase shares of our common stock as final compensation under the consulting agreement.
On March 18, 2024, Legacy TuHURA entered into a consulting agreement with Dr. Patricia Lawman in her individual capacity, for consulting services related to clinical strategy, technical consulting and other support systems related to the IFx-2.0 and IFx-3.0 clinical products. Through this consulting agreement, we pay Dr. Patricia Lawman $500 per hour for such services, with a total monthly fee not to exceed $25,000. This consulting agreement with Dr. Patricia Lawman terminates on April 1, 2025 unless otherwise extended by the parties.
113
TuHURA Note Financing
On April 2, 2024, K&V Investment One, LLC (“K&V Investment”), a holder of more than 5% of the fully diluted capital stock of the Company, participated in the TuHURA Note Financing and executed and delivered to Legacy TuHURA a subscription agreement, for $10.0 million in TuHURA convertible notes (the “K&V Investment Note”). In connection with the closing of the Kintara Merger, the K&V Investment Note converted into 3,157,059 shares of Legacy TuHURA common stock (as adjusted by the exchange ratio in the Kintara Merger), subject to the terms therein. K&V Investment’s subscription agreement provided for the initial funding of $500,000 on April 2, 2024, with the remaining $9.5 million funded in August 2024. In addition, and in connection with the TuHURA Note Financing, Legacy TuHURA issued a warrant to K&V Investment to purchase 1,315,441 shares of Legacy TuHURA common stock (as adjusted by the exchange ratio in the Kintara Merger).
TuHURA Series A Warrant Extension
Dr. Kiran Patel, a former director of Legacy TuHURA that resigned in connection with the closing of the Kintara Merger accepted a six-month extension to the expiration date of certain warrants to purchase common stock of TuHURA held by Dr. Patel. In connection therewith, Dr. Patel entered into a Warrant Amendment Agreement, effective August 9 2024, to extend the expiration date of the Series A Warrants for a period of six (6) months to February 12, 2025. Dr. Patel holds in the aggregate 94,611 Series A Warrants to purchase our common stock (as adjusted by the exchange ratio in the Kintara Merger).
Item 14. Principal Accounting Fees and Services.
On July 31, 2019, Marcum LLP (“Marcum”), independent registered public accounting firm, was appointed as Kintara's auditors. Following the closing of the Kintara Merger, our audit committee appointed Cherry Bekaert LLP (“Cherry Bekaert”) as our independent registered public accounting firm for the year ending December 31, 2024. Cherry Bekaert has served as Legacy TuHURA’s independent auditor since 2018.
The following table summarizes the audit fees billed by Cherry Bekaert’s in connection with their audit of our consolidated financial statements and for other services provided during the period following the closing of the Kintara Merger and for the years ended December 31, 2023. For a summary of the audit fees paid by Kintara for the fiscal year ended June 30, 2024, see Kintara’s Annual Report on Form 10-K filed October 7, 2024.
Fee Category |
|
2024 |
|
|
2023 |
|
||
Audit Fees(1) |
|
$ |
193,200 |
|
|
$ |
146,425 |
|
Tax Fees(2) |
|
$30,953 |
|
|
$10,500 |
|
||
All other Fees(3) |
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
||
Total Fees |
|
$ |
224,153 |
|
|
$ |
156,925 |
|
Audit Committee Pre-Approval Policy and Procedures
In accordance with applicable laws, rules and regulations, our audit committee charter and pre-approval policies established by the audit committee require that the audit committee review in advance and pre-approve all audit and permitted non-audit fees for services provided to us by our independent registered public accounting firm. The services performed by, and the fees to be paid to, Cherry Bekaert for the years ending December 31, 2024 and 2023 were approved by our audit committee.
114
Part IV
Item 15. Exhibits, Financial Statement Schedules.
The following documents are filed as part of this Annual Report on Form 10-K:
(a)(1) Financial Statements
The list of consolidated financial statements set forth in the accompanying Index to the Consolidated Financial Statements at page F-1 of this Annual Report on Form 10-K is incorporated herein by reference. Such consolidated financial statements are filed as part of this Annual Report on Form 10-K.
(a)(2) Financial Statement Schedules
All financial statement schedules have been omitted because they are not applicable, not required or the information required is shown in the financial statements or the notes thereto.
(a)(3) Exhibits
The following is a list of exhibits filed (or incorporated by reference herein) as part of this Annual Report on Form 10-K.
Exhibit |
|
Description |
|
|
|
2.1†† |
|
|
|
|
|
2.2†† |
|
|
|
|
|
3.1 |
|
|
|
|
|
3.2 |
|
|
|
|
|
4.1 |
|
|
|
|
|
4.2 |
|
|
|
|
|
4.3 |
|
|
|
|
|
4.4 |
|
|
|
|
|
4.5 |
|
|
|
|
|
115
4.6 |
|
|
|
|
|
4.7 |
|
|
|
|
|
4.8 |
|
|
|
|
|
4.9 |
|
|
|
|
|
4.10 |
|
|
|
|
|
4.11 |
|
|
|
|
|
4.12 |
|
|
|
|
|
4.13 |
|
|
|
|
|
4.14 |
|
|
|
|
|
4.15 |
|
|
|
|
|
4.16 |
|
|
|
|
|
4.17 |
|
|
|
|
|
4.18* |
|
|
|
|
|
10.1 |
|
|
|
|
|
10.2# |
|
|
|
|
|
10.3 |
|
|
|
|
|
10.4 |
|
10.5 |
|
116
|
|
|
10.6 |
|
|
|
|
|
10.7# |
|
|
|
|
|
10.8# |
|
|
|
|
|
10.9# |
|
|
|
|
|
10.10† |
|
|
|
|
|
10.11† |
|
|
|
|
|
10.12† |
|
|
|
|
|
10.13†† |
|
|
|
|
|
10.14 |
|
|
|
|
|
10.15 |
|
|
|
|
|
10.16 |
|
|
|
|
|
10.17# |
|
|
|
|
|
10.18#* |
|
TuHURA Biosciences, Inc. 2025 Non-Employee Director Compensation Program |
|
|
|
19.1* |
|
|
|
|
|
21.1 |
|
|
|
|
|
23.1* |
|
Consent of Cherry Bekaert LLP, independent registered public accounting firm. |
|
|
|
31.1* |
|
|
|
|
|
31.2 * |
|
|
|
|
|
117
32.1** |
|
|
|
|
|
97.1* |
|
|
|
|
|
101.INS |
|
XBRL Instance Document
|
101.SCH
|
|
SBRL Schema Document
|
101.CAL
|
|
Calculation Linkbase Document
|
101.DEF
|
|
Definition Linkbase Document
|
101.LAB
|
|
XBRL Label Linkbase Document
|
101.PRE
|
|
XBRL Presentation Linkbase Document
|
104 |
|
Cover Page Interactive Data File (formatted in Inline XBRL and contained in Exhibit 101) |
|
|
|
† Confidential treatment is requested for certain confidential portions of this exhibit pursuant to Rule 24b-2 under the Exchange Act. In accordance with Rule 24b-2, these confidential portions have been omitted from this exhibit and filed separately with the Securities and Exchange Commission.
†† Schedule has been omitted pursuant to Item 601(a)(5) of Regulation S-K. TuHURA hereby undertakes to furnish copies of any of the omitted schedules upon request by the Securities and Exchange Commission.
# Indicates a management contract or any compensatory plan, contract or arrangement.
* Filed herewith.
** This certification is being furnished solely to accompany this Report pursuant to 18 U.S.C. Section 1350, and is not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and is not to be incorporated by reference into any filing of the Registrant, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 16. Form 10-K Summary.
None.
118
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
TUHURA BIOSCIENCES, INC. |
|
|
|
|
Dated: March 31, 2025 |
By: |
/s/ James A. Bianco |
|
Name: |
James A. Bianco, M.D. |
|
Title: |
President and Chief Executive Officer |
KNOW ALL THESE PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints James A. Bianco, M.D. and Dan Dearborn and each of them, jointly and severally, his attorneys-in-fact, each with full power of substitution, for him in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each said attorneys-in-fact or his substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
SIGNATURE |
|
TITLE |
|
DATE |
|
|
|
|
|
/s/ James A. Bianco |
|
President, Chief Executive Officer and Director (Principal Executive Officer) |
|
March 31, 2025 |
James A. Bianco, M.D. Chief Executive Officer |
|
|
|
|
|
|
|
|
|
/s/ Dan Dearborn |
|
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
|
March 31, 2025 |
Dan Dearborn Chief Financial Officer |
|
|
|
|
|
|
|
|
|
/s/ James Manuso |
|
Director |
|
March 31, 2025 |
James Manuso, Ph.D. |
|
|
|
|
|
|
|
|
|
/s/ Alan List |
|
Director |
|
March 31, 2025 |
Alan List, M.D. |
|
|
|
|
|
|
|
|
|
/s/ George Ng |
|
Director |
|
March 31, 2025 |
George Ng |
|
|
|
|
|
|
|
|
|
/s/ Robert Hoffman |
|
Director |
|
March 31, 2025 |
Robert Hoffman
/s/ Craig Tendler______________________ Craig Tendler, M.D. |
|
Director |
|
March 31, 2025
|
119
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
TuHURA Biosciences, Inc. and Subsidiaries
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors
TuHURA Biosciences, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of TuHURA Biosciences, Inc. (the “Company”) as of December 31, 2024 and 2023, the related consolidated statements of operations, stockholders’ (deficit) equity, and cash flows for the years then ended, and the related notes to the consolidated financial statements (collectively the “financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
The Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company has recurring losses and negative cash flows from operations that raise substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which they relate.
Accounting for Reverse Recapitalization
As discussed in Note 1 of the accompanying financial statements, on October 18, 2024, the Nevada corporation formerly known as Kintara Therapeutics, Inc. (“Kintara”) consummated a merger transaction by and among Kintara, Kayak Mergeco, Inc., a Delaware corporation and direct wholly owned subsidiary of Kintara (“Merger Sub”), and TuHURA Biosciences, Inc., a Delaware corporation (“TuHURA”), pursuant to which, Merger Sub merged with and into TuHURA, with TuHURA surviving as a direct wholly owned subsidiary of Kintara and the surviving corporation of the merger (the “Merger”). Immediately following the Merger, Kintara changed its name to TuHURA Biosciences, Inc. The Merger was accounted for as a reverse recapitalization in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”), in which TuHURA was determined to be the accounting acquirer based upon the terms of the Merger and other certain factors.
We identified the accounting for the reverse recapitalization as a critical audit matter because of the complexity in the determination of the proper treatment of the transaction in accordance with U.S. GAAP, including judgments made by management to arrive at the proper conclusion. This required a high degree of auditor judgment and increased level of effort when performing audit procedures.
F-3
Our audit procedures related to the Company’s accounting for the reverse recapitalization included the following, among others:
/s/
We have served as the Company’s auditor since 2018.
March 31, 2025
F-4
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Assets |
|
|
|
|
|
|
||
Current Assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
Deposits, planned business acquisition (note 1) |
|
|
|
|
|
- |
|
|
Other current assets |
|
|
|
|
|
|
||
Total Current Assets |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Property and equipment, net |
|
|
|
|
|
|
||
Operating right-of-use assets |
|
|
|
|
|
|
||
Other noncurrent assets |
|
|
|
|
|
- |
|
|
Total Assets |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Liabilities and Stockholders' Equity (Deficit) |
|
|
|
|
|
|
||
Current Liabilities: |
|
|
|
|
|
|
||
Accounts payable and accrued expenses |
|
$ |
|
|
$ |
|
||
Derivative liability |
|
|
- |
|
|
|
|
|
Lease liabilities, current |
|
|
|
|
|
|
||
Total Current Liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Long-term Liabilities: |
|
|
|
|
|
|
||
Convertible notes payable, net |
|
|
- |
|
|
|
|
|
Lease liability, long term |
|
|
|
|
|
- |
|
|
Total Liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Stockholders' Equity (Deficit): |
|
|
|
|
|
|
||
Preferred Stock Series A (assumed in merger); $ |
|
|
|
|
|
- |
|
|
Preferred stock (Series A, A-1, and B) $ |
|
|
- |
|
|
|
|
|
Common stock, $ |
|
|
|
|
|
|
||
Additional paid in capital |
|
|
|
|
|
|
||
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
Total Stockholders' Equity (Deficit ) |
|
|
|
|
|
( |
) |
|
Total Liabilities and Stockholders' Equity (Deficit) |
|
$ |
|
|
$ |
|
||
F-5
|
|
Year Ended December 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Research and development expenses |
|
$ |
|
|
$ |
|
||
Acquired in-process research and development ("IPR&D") |
|
|
- |
|
|
|
|
|
General and administrative expenses |
|
|
|
|
|
|
||
Operating Loss |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Other (Expense) Income: |
|
|
|
|
|
|
||
Employee Retention Tax Credit |
|
|
- |
|
|
|
|
|
Grant income |
|
|
|
|
|
|
||
Interest expense |
|
|
( |
) |
|
|
( |
) |
Interest income |
|
|
|
|
|
|
||
Change in fair value of derivative liability |
|
|
( |
) |
|
|
- |
|
Total Other (Expense) Income |
|
|
( |
) |
|
|
|
|
Net Loss |
|
$ |
( |
) |
|
$ |
( |
) |
Series A Preferred cash dividend |
|
|
( |
) |
|
|
- |
|
Deemed dividend on warrant modifications |
|
|
( |
) |
|
|
- |
|
Net Loss attributable to common stockholders |
|
$ |
( |
) |
|
$ |
( |
) |
Net Loss per share, basic and diluted |
|
$ |
( |
) |
|
$ |
( |
) |
Weighted-average shares outstanding, basic and diluted |
|
|
|
|
|
|
||
F-6
TUHURA BIOSCIENCES, INC AND SUBSIDIARIES
Consolidated statements of stockholders’ equity (deficit)
For the years ended December 31, 2024, and 2023
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|||||||
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
Accumulated |
|
|
Stockholders' |
|
||||||||||
|
Preferred Stock |
|
|
Common Stock |
|
|
Paid in |
|
|
Equity |
|
|
Equity |
|
|||||||||||||
|
Shares |
|
|
Dollars |
|
|
Shares |
|
|
Dollars |
|
|
Capital |
|
|
(Deficit) |
|
|
(Deficit) |
|
|||||||
Balances at January 1, 2023 |
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Retroactive application of reverse recapitalization |
|
( |
) |
|
$ |
|
|
|
( |
) |
|
$ |
|
|
$ |
( |
) |
|
$ |
- |
|
|
$ |
- |
|
||
Preferred stock (Series A ratio |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Recast balances, beginning of period (applying |
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Issuance of common shares for asset acquisition |
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
|
||||
Shares repurchased |
|
( |
) |
|
|
( |
) |
|
|
- |
|
|
|
- |
|
|
|
( |
) |
|
|
- |
|
|
|
( |
) |
Stock compensation expense |
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
|
||
Net loss |
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
( |
) |
|
|
( |
) |
Balances at December 31, 2023 |
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
|||||
The accompanying notes to the consolidated financial statements are an integral part of this statement. |
F-7 |
TUHURA BIOSCIENCES, INC AND SUBSIDIARIES
Consolidated statements of stockholders’ equity (deficit) continued
For the years ended December 31, 2024, and 2023
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|||||||
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
Accumulated |
|
|
Stockholders' |
|
||||||||||
|
Preferred Stock |
|
|
Common Stock |
|
|
Paid in |
|
|
Equity |
|
|
Equity |
|
|||||||||||||
|
Shares |
|
|
Dollars |
|
|
Shares |
|
|
Dollars |
|
|
Capital |
|
|
(Deficit) |
|
|
(Deficit) |
|
|||||||
Balances at January 1, 2024 |
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Conversion of preferred stock to common stock |
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
||
Issuance of common stock upon settlement of |
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
|
||||
Reclassification of the derivative liability associated |
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
|
||
Common stock and preferred stock (assumed from |
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
- |
|
|
|
( |
) |
||||
Transaction costs in connection with the reverse |
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
( |
) |
|
|
- |
|
|
|
( |
) |
Issuance of common shares, net of offering costs |
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
|
||||
Issuance of common shares for equity issuance and |
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
|
||||
Issuance of common shares for warrants exercised |
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
|
||||
Stock options exercised, cash and cashless |
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
|
||||
Stock compensation expense |
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
|
||
Fair value of warrants associated with convertible notes |
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
|
||
Deemed dividend on warrant modifications |
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
( |
) |
|
|
- |
|
|
Series A Preferred Stock cash dividend |
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
( |
) |
|
|
( |
) |
Net loss |
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
( |
) |
|
|
( |
) |
Balances at December 31, 2024 |
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||||||
F-8
TUHURA BIOSCIENCES, INC AND SUBSIDIARIES
Consolidated statements of cash flows
For the years ended December 31, 2024, and 2023
|
|
Years ended |
|
|||||
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Cash flows from Operating activities: |
|
|
|
|
|
|
||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Adjustments to reconcile net loss to cash |
|
|
|
|
|
|
||
used in operating activities: |
|
|
|
|
|
|
||
Stock compensation expense |
|
|
|
|
|
|
||
Depreciation and amortization |
|
|
|
|
|
|
||
Write-off of in-process R&D |
|
|
- |
|
|
|
|
|
Amortization of debt discount |
|
|
|
|
|
|
||
Change in fair value of derivative liability |
|
|
|
|
|
- |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
||
Other current assets |
|
|
( |
) |
|
|
( |
) |
Other noncurrent assets |
|
|
|
|
|
|
||
Accounts payable and accrued expenses |
|
|
|
|
|
|
||
Net cash flows from operating activities |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Cash flows from investing activities: |
|
|
|
|
|
|
||
Cash paid for asset acquisition |
|
|
- |
|
|
|
( |
) |
Deposits, planned business acquisition |
|
|
( |
) |
|
|
- |
|
Purchase of property and equipment |
|
|
( |
) |
|
|
( |
) |
Net cash flows from investing activities |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Cash flows from financing activities: |
|
|
|
|
|
|
||
Shares repurchased |
|
|
- |
|
|
|
( |
) |
Proceeds from convertible notes payable |
|
|
|
|
|
|
||
Proceeds from issuance of common stock |
|
|
|
|
|
- |
|
|
Proceeds from stock options exercised |
|
|
|
|
|
- |
|
|
Proceeds from warrants exercised |
|
|
|
|
|
- |
|
|
Payment of offering costs associated with issuance of common stock |
|
|
( |
) |
|
|
- |
|
Payment of merger transaction costs |
|
|
( |
) |
|
|
- |
|
Payment of debt issuance costs |
|
|
( |
) |
|
|
- |
|
Payment of net liabilities assumed in reverse recapitalization |
|
|
( |
) |
|
|
- |
|
Net cash flows from financing activities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Net change in cash and cash equivalents |
|
|
|
|
|
( |
) |
|
Cash and cash equivalents at the beginning of the year |
|
|
|
|
|
|
||
Cash and cash equivalents at the end of the year |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Supplemental non-cash activity |
|
|
|
|
|
|
||
Shares issued and reserved for asset acquisition |
|
$ |
- |
|
|
$ |
|
|
Right-of-use asset recognized in exchange for operating lease obligations |
|
|
|
|
|
- |
|
|
Debt issuance costs not yet paid |
|
|
- |
|
|
|
|
|
Net liabilities assumed in reverse recapitalization not yet paid |
|
|
|
|
|
- |
|
|
Merger transaction costs not yet paid |
|
|
|
|
|
- |
|
|
Derivative liability associated with make-whole premium |
|
|
|
|
|
|
||
Fair value of warrants associated with convertible notes payable |
|
|
|
|
|
- |
|
|
Deemed dividend on warrant modifications |
|
|
|
|
|
- |
|
|
Issuance of common stock for placement agent fees |
|
|
|
|
|
- |
|
|
Preferred Series A cash dividend not yet paid |
|
|
|
|
|
- |
|
|
Fair value of contingent value rights associated with reverse recapitalization |
|
|
|
|
|
- |
|
|
Fair value of warrants issued to financial advisor |
|
|
|
|
|
- |
|
|
Shares issued upon settlement of convertible notes |
|
|
|
|
|
- |
|
|
F-9
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
Note 1—Description of business
TuHURA Biosciences, Inc., a Nevada corporation (the “Company”), is a clinical stage immuno-oncology company developing novel personalized cancer vaccine product candidates designed to overcome primary resistance to immunotherapies like checkpoint inhibitors. The Company has entered into a Special Protocol Assessment agreement with the FDA for a single Phase 3 randomized placebo and injection controlled trial for IFx-2.0, the Company’s lead personalized cancer vaccine product candidate, as adjunctive therapy to pembrolizumab (Keytruda®) in the first line treatment of patients with advanced or metastatic Merkel cell carcinoma who are checkpoint inhibitor naïve utilizing the FDA’s accelerated approval pathway. The Company is also developing novel bi-functional antibody drug conjugates, or ADCs, targeting myeloid derived suppressor cells, or MDSCs, to modulate their immunosuppressive effects on the tumor microenvironment to overcome acquired resistance to immunotherapies and IFx-3.0 as an innate immune agonist candidate for intravenous or autologous whole cell administration for blood related cancers.
Reverse Merger with Kintara Therapeutics, Inc.
On October 18, 2024 (the “Closing Date”), the Nevada corporation formerly known as Kintara Therapeutics, Inc. (“Kintara”) consummated a previously announced merger transaction in accordance with the terms of that certain Agreement and Plan of Merger, dated as of April 2, 2024 (the “Merger Agreement”), by and among Kintara, Kayak Mergeco, Inc., a Delaware corporation and direct wholly owned subsidiary of Kintara (“Merger Sub”), and TuHURA Biosciences, Inc., a Delaware corporation (“TuHURA”), pursuant to which, Merger Sub merged with and into TuHURA, with TuHURA surviving as a direct wholly owned subsidiary of Kintara and the surviving corporation of the merger (the “Merger”). In connection with the completion of the Merger, effective on the Closing Date, Kintara effected a
Under the terms of the Merger, immediately prior to the effective time of the Merger, shares of TuHURA’s preferred stock were converted into shares of TuHURA’s common stock and all of the convertible notes issued in TuHURA’s private placement (the “TuHURA Note Financing”) were converted into shares of TuHURA common stock pursuant to the terms therein. At the effective time of the Merger, (i) Kintara issued an aggregate of approximately
The issuance of the shares of Kintara’s common stock to the former stockholders of TuHURA was registered with the SEC on Kintara’s Registration Statement on Form S-4 (File No. 333-279368), as amended.
The shares of Kintara’s common stock listed on the Nasdaq Capital Market, previously trading through the close of business on Thursday, October 17, 2024 under the ticker symbol “KTRA,” commenced trading on the Nasdaq Capital Market on a post-Reverse Stock Split adjusted basis and post-Merger basis under the ticker symbol “HURA” on Friday, October 18, 2024.
In connection with the Merger, Kintara entered into a Contingent Value Rights Agreement (the “CVR Agreement”) with Equiniti Trust Company, LLC, pursuant to which the Kintara common stock holders and Kintara common stock warrant holders of record as of immediately prior to the consummation of the Merger and Reverse Stock Split received one contingent value right (a “CVR”) for each outstanding share of common stock held by such stockholder (or, in the case of warrants, each share of common stock for which such warrant is exercisable into). Pursuant to the CVR Agreement, upon the achievement of (i) Kintara enrolling a minimum of ten cutaneous metastatic breast cancer patients in a study to determine whether a dose of Kintara’s REM-001 lower than 1.2 mg/kg elicits a treatment effect similar to that seen in prior studies of REM-001 at the 1.2 mg/kg dose and (ii) such patients enrolled in the study complete eight weeks of follow-up, in each case, on or before December 31, 2025, as set forth in the CVR Agreement (the holders of CVRs are entitled, in the aggregate, to receive approximately
F-10
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
Basis of presentation
The merger was accounted for as a reverse recapitalization under which the historical financial statements of the Company prior to the Merger are the historical financial statements of the accounting acquirer, Legacy TuHURA. All share, per share and related information presented in the consolidated financial statements and notes prior to the Merger has been retroactively adjusted to reflect the Exchange Ratio and Reverse Stock Split for all periods presented. See note 6 – Merger with Kintara Therapeutics.
Plan of merger with Kineta, Inc.
On December 11, 2024, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement” or “Proposed Transaction”) by and among Kineta, Inc., a Delaware corporation (“Kineta”), Hura Merger Sub I, Inc., a Delaware corporation and a direct wholly-owned subsidiary of TuHURA (“Merger Sub I”), Hura Merger Sub II, LLC, a Delaware limited liability company and direct wholly-owned subsidiary of TuHURA (“Merger Sub II,” and together with Merger Sub I, the “Merger Subs”), and Craig Philips, solely in his capacity as the representative, agent and attorney-in-fact of the stockholders of Kineta (the “Stockholders Representative”). Each capitalized term used herein but not otherwise defined has the meaning given to it in the Merger Agreement.
Pursuant to the terms of the Merger Agreement, among other things and subject to the terms and conditions set forth therein, Merger Sub I will (a) merge with and into Kineta (the “First Merger”), with Kineta being the surviving corporation of the First Merger, also known as the “Surviving Entity”; and (b) immediately following the First Merger and as part of the same overall transaction as the First Merger, the Surviving Entity will merge with and into Merger Sub II (the “Second Merger” and, together with the First Merger, the “Mergers” ), with Merger Sub II being the surviving company of the Second Merger, also known as the “Surviving Company.” The Mergers are intended to qualify as a tax-free reorganization for U.S. federal income tax purposes.
The Registration Statement on S-4 for the planned merger was filed with the SEC on February 7, 2024.
Merger Consideration
Under the terms of the merger agreement, upon the completion of the Proposed Transaction, Kineta stockholders will receive their pro rata share (based on the number of Kineta fully diluted shares held by them) of aggregate merger consideration consisting of a combination of cash and shares of TuHURA common stock. The cash component of the aggregate merger consideration will be a base cash amount of $
In connection with the merger agreement, TuHURA and Kineta entered into a Clinical Trial Funding Agreement under which TuHURA agreed to continue to fund clinical trial expenses for KVA12123 in an amount of up to $
The merger agreement has been unanimously approved by the boards of directors of both companies and is subject to Kineta stockholder approval. The completion of the Proposed Transaction is also subject to the satisfaction or waiver of certain other conditions, such as the approval by TuHURA’s stockholders of an increase in the number of authorized shares of TuHURA common stock, Kineta’s working capital deficit not exceeding $
F-11
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
Other Terms of the Merger Agreement
The Merger Agreement contains customary representations, warranties and covenants made by Kineta and TuHURA, including covenants relating to obtaining the requisite approvals of the stockholders of Kineta and TuHURA, indemnification of directors and officers and Kineta’s and TuHURA’s conduct of their respective businesses between the date of signing of the Merger Agreement and the Closing. The parties have agreed to use reasonable best efforts to take all actions necessary to consummate the Mergers, including for Kineta to hold a meeting of its stockholders to vote on proposals related to the Mergers and for TuHURA to hold a meeting of its stockholders to vote on a proposal to increase the authorized shares of TuHURA to
July 2024 Private Placement
In connection with the Company’s entrance into the Exclusivity Agreement, on July 3, 2024, the Company completed a private placement of its common stock to an existing investor under which the investor paid $
Note 2—Summary of significant accounting policies
Basis for Consolidation - The consolidated financial statements of the Company have been prepared in accordance with United States Generally Accepted Accounting Principles (“U.S. GAAP”) and are presented in United States dollars. The functional currency of the Company and each of its subsidiaries is the United States dollar.
The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, Adgero Biopharmaceuticals Holdings Inc., Adgero Biopharmaceuticals, Inc., Veterinary Oncology Services, and TuHURA (Delaware). All intercompany balances and transactions have been eliminated in consolidation.
The principal accounting policies applied in the preparation of these consolidated financial statements are set out below and have been consistently applied to all periods presented.
Accounting Estimates – The preparation of consolidated financial statements in conformity with generally accepted accounting principles in the United States of America (“U.S. GAAP”) requires management to make estimates and assumptions that affect various amounts reported in consolidated financial statements and accompanying notes. Actual results could differ from those estimates.
Merger Transaction Costs – Deferred offering costs consist of direct legal, accounting, and other fees and costs directly related to the Merger with Kintara (See note 1 and note 6). The Company capitalized merger transaction costs and recorded as a direct reduction of stockholders’ equity.
Property and Equipment – Property and equipment are carried at cost, less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over the estimated useful lives of the assets (generally to
Lease Accounting – The Company recognizes right-of-use lease assets and corresponding liabilities arising from leasing activities over the requisite lease period.
Income Taxes – Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740), which enhances the income tax disclosure requirements for public entities on an annual
F-12
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
basis. Under ASU 2023-09, public entities will be required to disclose in their rate reconciliation, on an annual basis, both percentages and amounts in their reporting currency for certain categories in a tabular format, with accompanying qualitative disclosures. The amendments in ASU 2023-09 are effective fiscal years beginning after December 31, 2024, and early adoption is permitted. The Company does not believe that the adoption of ASU 2023-09 will have a material impact on its consolidated financial statements.
Grant Income - In April 2021, the Company received approval from the Department of Health and Human Services for a $
Research and Development Expenses – Research and development consists of expenses incurred in connection with the discovery and development of product candidates. The Company expenses research and development costs as incurred.
Acquired In-Process Research and Development - Acquired in-process research and development expenses consist of existing research and development projects at the time of the acquisition. Projects that qualify as IPR&D assets represent those that have not yet reached technological feasibility and had no alternative future use, which resulted in a write-off of these IPR&D assets to acquired in-process research and development expenses in our consolidated statements of operations.
Concentration of Credit Risk – The Company maintains cash balances in domestic financial institutions. These balances are insured by the Federal Deposit Insurance Corporation up to $
Fair Value of Financial Instruments - ASC 820, Fair Value Measurement, establishes a fair value hierarchy for instruments measured at fair value that distinguishes between assumptions based on market data (observable inputs) and the Company’s own assumptions (unobservable inputs). Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the inputs that market participants would use in pricing the asset or liability, and are developed based on the best information available in the circumstances.
ASC 820 identifies fair value as the exchange price, or exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As a basis for considering market participant assumptions in fair value measurements, ASC 820 establishes a three-tier fair value hierarchy that distinguishes between the following:
To the extent that the valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized in Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. See Note 5 for more information related to the Company’s Level 3 fair value measurement.
F-13
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
The carrying values reported in the Company’s balance sheets for cash and cash equivalents, other current assets, accounts payable, and accrued expenses are reasonable estimates of their fair values due to the short-term nature of these items.
Derivative Financial Instruments – The Company evaluates all of its agreements to determine if such instruments have derivatives or contain features that qualify as embedded derivatives. The Company accounts for certain make-whole features that are associated with convertible notes as derivative liabilities at fair value and adjusts the instruments to their fair value at the end of each reporting period. Derivative financial liabilities are initially recorded at fair value, with gains and losses arising from changes in the fair value recognized in other income (expense) in the accompanying consolidated statements of operations for each reporting period while such instruments are outstanding. The embedded derivative liabilities are valued using a probability-weighted expected return method (“PWERM”). The critical inputs used to value the PWERM are a discount rate, the estimated make-whole interest payments for various settlement scenarios and the probability of each settlement scenario. If the Company repays the noteholders or if, during the next round of financing, the noteholders convert the debt into equity, the derivative financial liabilities will be de-recognized and reclassified to the consolidated statements of stockholders’ (deficit) equity on that date. Derivative instrument liabilities are classified in the balance sheet as current or non-current based on whether net-cash settlement of the derivative instrument could be required within 12 months of the balance sheet date.
Debt Discount and Debt Issuance Costs- Debt issuance costs are deferred and presented as a reduction to the convertible note payable. The initial fair value of the derivative liability on the make-whole premium is treated as a debt discount. Debt discount and debt issuance costs are amortized using the effective interest rate method over the term of the convertible promissory note. Amortization of debt discount and debt issuance costs are included within interest expense in the consolidated statements of operations.
Warrants – The Company has issued warrants to investors on debt and equity raises and for services provided by non-employees. In accordance with ASC Topic 470-20-25, when the Company issues debt with warrants, the Company treats the warrants as a debt discount, recorded as a contra-liability against the debt, and amortizes the balance over the life of the underlying debt as amortization of the discount as interest expense in the statements of operations. The offset to the contra-liability is recorded as additional paid-in capital in the Company’s balance sheets if the warrants on the debt are not treated as a derivative or as liability warrants. The Company determines the fair value of the warrants at issuance using the Black-Scholes option pricing model.
Stock Compensation Expense – The Company accounts for stock-based awards to employees and nonemployees using the fair value-based method to determine compensation for all arrangements where shares of stock or equity instruments are issued for compensation. Fair value of each common stock option is estimated on the date of grant using the Black-Scholes valuation model. The Black-Scholes model uses assumptions for expected volatility, expected dividends, expected term, and the risk-free interest rate. Expected volatility is based on historical volatility of a peer group’s common stock and other factors estimated over the expected term of the options. The expected term of the options granted is derived using the “simplified method” which computes expected term as the average of the sum of the average vesting term plus the contract term. The risk-free rate is based on the U.S. Treasury yield.
Common Stock Valuation – We are required to estimate the fair value of the common stock underlying our equity awards when performing fair value calculations. Prior to our shares trading on the Nasdaq Capital Markets on October 18, 2024, the fair value of the common stock underlying our equity awards was determined on each grant taking into account input from management and the pricing offered in our equity raises. All options to purchase shares of our common stock are intended to be granted with an exercise price per share no less than the fair value per share of our common stock underlying those options on the date of grant, based on the information known to us on the date of grant. In the absence of a public trading market for our common stock, on each grant date we develop an estimate of the fair value of our common stock in order to determine an exercise price for the option grants. Our determinations of the fair value of our common stock were made by considering the prices of preferred stock sold to investors in arm’s length transactions and the rights, preferences and privileges of our preferred stock relative to those of our common stock.
Business Combinations and Asset Acquisitions – We account for acquired businesses using the acquisition method of accounting, which requires that the assets acquired, and liabilities assumed be recorded at the date of acquisition at their respective fair values if the acquisition meets the definition of a business combination. If the acquisition does not meet the definition of a business combination, then it is accounted for as an asset acquisition and the purchase consideration is allocated to the acquired assets.
F-14
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
ASC 805, Business Combinations, provides a model for determining whether an acquisition represents a business combination. In order to be a business, the integrated set of activities of the acquired entity needs to have an input and a substantive process that together significantly contribute to the ability to create outputs. The acquired entity must also pass the “Screen Test” which involves determining whether the acquisition represents an in-substance asset acquisition based on whether the fair value of the gross assets acquired is “substantially all” concentrated in a single asset or group of similar assets. This evaluation excludes certain acquired assets such as cash, deferred taxes, and goodwill associated with deferred taxes, but includes all other gross assets, including any consideration transferred in excess of the identified assets.
Segment data - In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. ASU 2023-07 updates reportable segment disclosure requirements primarily through enhanced disclosures about significant segment expenses. The Company has adopted ASU 2023-07, and the guidance did not have a material impact on the Company's consolidated financial statements. The Company operates in one reportable segment, which includes all activities related to advancing therapies for cancer treatment. The determination of a single reportable segment is consistent with the consolidated financial information regularly provided to the Company’s chief operating decision maker (CODM), which is its , who reviews and evaluates consolidated net loss for purposes of assessing performance, making operating decisions, allocating resources and planning and forecasting for future periods. The measure of segment assets is reported on the balance sheet as total assets. There is
Net loss per share - Basic net loss per share is calculated by dividing the net loss by the weighted-average number of shares of common stock outstanding during the period, without consideration for common stock equivalents. Diluted net loss per share is the same as basic net loss per share, since the effects of potentially dilutive securities are antidilutive given the Company has reported net losses for each period presented.
Note 3—Liquidity and management’s plans
The Company has been engaged in research and development activities related to ImmuneFx, the Company’s patented product, which will require additional investment until revenue-generating activities can begin.
The Company has historically incurred negative cash flows from operations.
For the year ended December 31, 2024, the Company incurred $
The Company expects to raise cash through the sale of preferred shares, common shares, debt issuances, obtaining grants, or commercial partnerships. However, there can be no assurance that any fundraising will be achieved or on commercially reasonable terms, if at all. As such, there is substantial doubt about the Company’s ability to continue as a going concern for the next 12 months from date that the financial statements were available to be issued.
F-15
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
Note 4—Net loss per share
Basic and diluted net loss per share attributable to common stockholders was calculated as follows:
|
|
Years Ended December 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Numerator: |
|
|
|
|
|
|
||
Net loss attributable to common stockholders |
|
$ |
( |
) |
|
$ |
( |
) |
Denominator: |
|
|
|
|
|
|
||
Weighted-average common shares outstanding - basic and diluted |
|
|
|
|
|
|
||
Net loss per share attributable to common shareholders - basic and diluted |
|
$ |
( |
) |
|
$ |
( |
) |
Common stock warrants in the amount of
The Company’s potential dilutive securities have been excluded from the computation of diluted net loss per share as the effect would be to reduce the net loss per share. Therefore, the weighted-average number of common shares outstanding used to calculate both basic and diluted net loss per share attributable to common stockholders is the same.
|
|
As of December 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Preferred Series A (as converted) |
|
|
|
|
|
|
||
Preferred Series A-1 (as converted) |
|
|
|
|
|
|
||
Preferred Series B (as converted) |
|
|
|
|
|
|
||
Convertible Notes (as converted) |
|
|
|
|
|
|
||
Stock options issued and outstanding |
|
|
|
|
|
|
||
Unvested restricted stock units |
|
|
|
|
|
|
||
Warrants |
|
|
|
|
|
|
||
Total |
|
|
|
|
|
|
||
Note 5—Fair value measurements
The following tables present information about financial assets and liabilities measured at fair value on a recurring basis and indicate the level of the fair value hierarchy utilized to determine such fair values:
|
|
December 31, 2023 |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Derivative Liability |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Total Liabilities |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
There were no transfers between the Level 1, Level 2 or Level 3 categories during the years ended December 31, 2024 and 2023.
F-16
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
Derivative Liability
The following table presents activity for the Derivative Liability that were measured at fair value using significant unobservable Level 3 inputs during the years ended December 31, 2024 and, 2023:
|
|
Derivative |
|
|
Balance as of January 1, 2023 |
|
$ |
|
|
Additions (make-whole provision in convertible notes agreement) |
|
|
|
|
Change in fair value |
|
|
|
|
Balance as of December 31, 2023 |
|
$ |
|
|
Additions (make-whole provision in convertible notes agreement) |
|
|
|
|
Change in fair value |
|
|
|
|
Reclassification to additional paid-in capital |
|
|
( |
) |
Balance as of December 31, 2024 |
|
$ |
|
|
Note 6 – Merger with Kintara Therapeutics
Under the terms of the Merger, immediately prior to the effective time of the Merger, shares of the Company’s preferred stock were converted into shares of Company common stock and all of the Notes issued by the Company were converted into shares of Company common stock pursuant to the terms therein. At the effective time of the Merger, (i) Kintara issued an aggregate of approximately
The Merger is accounted for as a reverse recapitalization. Immediately after the merger, there were
|
|
October 18, |
|
|
|
|
2024 |
|
|
Cash and cash equivalents |
|
$ |
|
|
Prepaid and other assets |
|
|
|
|
Accounts payable and accrued expenses |
|
|
( |
) |
Total net liabilities assumed |
|
|
( |
) |
Plus: merger transaction costs |
|
|
( |
) |
Total net liabilities assumed plus merger transaction costs |
|
$ |
( |
) |
Accounting treatment on the Contingent Value Rights “CVR” agreement
Based on management’s analysis, the CVRs were identified as freestanding financial instruments and determined to be indexed to Kintara’s own stock, as they are to be settled in Kintara Common Stock. Further, the CVR financial instruments are not mandatorily redeemable as the instruments do not require Kintara to redeem them for cash or other assets at a fixed or determinable date, or upon an event that is certain to occur and the CVRs do not represent an unconditional obligation requiring Kintara to redeem the instruments. The CVRs do not represent outstanding shares of Kintara Common Stock, and the CVRs do not obligate Kintara to buy back some or all of its shares. As such, the CVRs are not precluded from being classified within equity. Given the CVRs are initially being recorded
F-17
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
within Equity, if the CVR Milestone were to be achieved, the Company would issue additional Common Stock, thereby resulting in a reclass of the CVRs from Additional paid-in capital—CVRs to Common Stock and Additional paid-in capital. As a result, the accounting for the CVR is determined to have zero net effect on total equity within the consolidated balance sheet as of December 31, 2024.
Note 7—Other current assets
Other current assets consist of the following as of December 31, 2024, and 2023:
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Employee Retention Tax Credit |
|
$ |
|
|
$ |
|
||
NIH Grant Receivable |
|
|
|
|
|
- |
|
|
Clinical trial deposit |
|
|
|
|
|
- |
|
|
Other current assets |
|
|
|
|
|
|
||
|
|
$ |
|
|
$ |
|
||
Note 8—Property and equipment, net
Property and equipment, net consists of the following as of December 31, 2024, and 2023:
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Furniture and fixtures |
|
$ |
|
|
$ |
|
||
Leasehold improvements |
|
|
|
|
|
|
||
Machinery and office equipment |
|
|
|
|
|
|
||
Software |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Less accumulated depreciation and amortization |
|
|
( |
) |
|
|
( |
) |
|
|
$ |
|
|
$ |
|
||
Depreciation and amortization of property and equipment totaled approximately $
Note 9—Accounts payable and accrued expenses
Accounts payable and accrued expenses consist of the following as of December 31, 2024, and 2023:
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Trade accounts payable |
|
$ |
|
|
$ |
|
||
Accrued compensation |
|
|
|
|
|
|
||
Other accrued expenses |
|
|
|
|
|
|
||
|
|
$ |
|
|
$ |
|
||
Note 10—Convertible promissory notes
On various dates beginning on December 11, 2023 through September 18, 2024, the Company completed a private placement in which the Company issued Convertible Promissory Notes (the “Notes”) with various entities at various amounts for an aggregate of $
F-18
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
(the “2024 Warrants”) in the event they subscribe to purchase Notes in the aggregate principal amount of more than $
Conversion feature under reverse public merger transaction
Under a reverse public merger transaction, the Notes convert at the sum of (a) the outstanding principal balance and unpaid accrued interest at the time of the transaction, plus (b) a Make-Whole Amount premium, defined in the Notes as additional interest to be incurred until the next period end date as defined in the Notes, divided by a conversion price equal to $
The Company evaluated the terms of the Notes for embedded conversion features in accordance with ASC 815-15-25 and determined that the conversion features meet the definition of an embedded derivative liability that is required to be bifurcated from the host instrument and measured at fair value, with subsequent changes in fair value recognized in the consolidated statement of operations. Management used a scenario-based analysis to estimate the fair value of the bifurcated embedded derivative liability at issuance of the Notes. The Company recognized debt discount of $
Warrants issued in connection with convertible notes
The 2024 Warrants were identified as freestanding financial instruments and determined to be indexed to the Company’s own stock. Further, the 2024 Warrants were not precluded from being classified within equity. As such, the proceeds received upon issuing the Notes were first allocated to the fair value of the bifurcated embedded derivative with the remainder allocated to the debt host instrument and 2024 Warrants (within additional paid in capital) on a relative fair value basis. Subsequent fair value measurement is not required as long as the instrument continues to be classified in equity. The Company determined that the fair value of the 2024 Warrants in connection with Notes issued amounted to $
Convertible notes and debt discount
Convertible notes issued (including accrued interest) outstanding, and converted are as follows:
|
|
Convertible notes |
|
|
Balance as of January 1, 2023 |
|
$ |
|
|
Issuance of convertible notes payable |
|
|
|
|
Interest expense |
|
|
|
|
Balance as of December 31, 2023 |
|
$ |
|
|
Issuance of convertible notes payable |
|
|
|
|
Interest expense |
|
|
|
|
Converted to common stock |
|
|
( |
) |
Balance as of December 31, 2024 |
|
$ |
|
|
Debt discount related to the convertible notes are as follows:
F-19
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
|
|
Debt discount |
|
|
Balance as of January 1, 2023 |
|
$ |
|
|
Debt issue costs |
|
|
( |
) |
Debt discount associated with make-whole features recognized |
|
|
( |
) |
Amortized to interest expense |
|
|
|
|
Balance as of December 31, 2023 |
|
$ |
( |
) |
Debt issue costs |
|
|
( |
) |
Debt discount associated with make-whole features recognized |
|
|
( |
) |
Debt discount associated with warrants recognized |
|
|
( |
) |
Amortized to interest expense |
|
|
|
|
Reclassified to additional paid-in capital upon conversion of convertible notes payable |
|
|
|
|
Balance as of December 31, 2024 |
|
$ |
|
|
Note 11—Income taxes
Income taxes are provided for the tax effects of transactions reported in the financial statements and consist of taxes currently due. Deferred taxes relate to differences between the basis of assets and liabilities for financial and income tax reporting which will be either taxable or deductible when the assets or liabilities are recovered or settled.
The components of the provision for income taxes are as follows:
|
|
2024 |
|
|
2023 |
|
||
Current provision |
|
|
|
|
|
|
||
Federal |
|
|
|
|
|
|
||
State |
|
|
|
|
|
|
||
Total current provision |
|
|
|
|
|
|
||
Deferred provision |
|
|
|
|
|
|
||
Federal |
|
|
|
|
|
|
||
State |
|
|
|
|
|
|
||
Total current provision |
|
|
|
|
|
|
||
Total provision for income taxes |
|
|
|
|
|
|
||
For the years ended December 31, 2024 and 2023, the loss before income taxes was $
|
|
2024 |
|
|
2023 |
|
||
U.S. statutory rate |
|
|
% |
|
|
% |
||
State taxes, net of federal |
|
|
% |
|
|
% |
||
Change in valuation allowance |
|
|
% |
|
|
- |
% |
|
Return to provision - 2023 Tax Free Reorganization |
|
|
- |
% |
|
|
% |
|
Return to provision - Other |
|
|
- |
% |
|
|
% |
|
R&D Credit |
|
|
- |
% |
|
|
% |
|
Other permanent differences |
|
|
- |
% |
|
|
% |
|
Effective tax rate |
|
|
- |
% |
|
|
% |
|
The Company made an acquisition in 2023. The transaction was complex and when preparing the tax provision for the year ended December 31, 2023, the Company made the assumption that the acquisition was taxable asset acquisition for US federal
F-20
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
income tax purposes when preparing its initial estimates. The Company booked a deferred tax asset for the intangible tax basis assumed to be acquired in a deemed asset acquisition of $
The components for the Company's deferred tax assets and liabilities as of December 31, 2024 and 2023, were as follows:
|
|
2024 |
|
|
2023 |
|
||
Deferred tax assets: |
|
|
|
|
|
|
||
Net operating loss carryforwards |
|
$ |
|
|
$ |
|
||
Stock compensation expense |
|
|
|
|
|
|
||
Accrued payroll |
|
|
|
|
|
|
||
Section 174 R&D |
|
|
|
|
|
|
||
Intangible assets |
|
|
|
|
|
|
||
Lease liability |
|
|
|
|
|
|
||
Depreciation |
|
|
|
|
|
|
||
Capital loss carryforward |
|
|
|
|
|
|
||
R&D credits |
|
|
|
|
|
|
||
Total gross deferred tax assets |
|
$ |
|
|
$ |
|
||
Less valuation allowance |
|
|
( |
) |
|
|
( |
) |
Net deferred tax assets |
|
$ |
|
|
$ |
|
||
Deferred tax liabilities |
|
|
|
|
|
|
||
Debt discount |
|
$ |
|
|
$ |
( |
) |
|
Accrued expense |
|
|
( |
) |
|
|
|
|
ROU asset |
|
|
( |
) |
|
|
( |
) |
Total deferred tax liabilities |
|
$ |
( |
) |
|
$ |
( |
) |
Total deferred tax assets / (liabilities) |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
The Company has Federal and State net operating loss (“NOLs”) carryforwards of approximately $
The utilization of the Company’s net operating loss carryforwards and research tax credit carryovers could be subject to annual limitations under Section 382 and 383 of the Internal Revenue Code of 1986, as amended (the “Code”), and similar state tax provisions, due to ownership change limitations that may have occurred previously or that could occur in the future. These ownership changes limit the amount of net operating loss carryforwards and other deferred tax assets that can be utilized to offset future taxable income and tax, respectively. In general, an ownership change, as defined by Section 382 and 383 of the Code, results from transactions increasing ownership of certain stockholders or public groups in the stock of the corporation by more than 50 percent points over a three-year period.
The acquisition of Kintara by TuHURA Delaware caused an ownership change with respect to the Kintara net operating losses. The Company is in the process of determining what the Section 382 limitation is with respect to the Kintara net operating losses for this ownership change and any prior ownership changes that will limit the Company's ability to utilize the net operating losses under Section 382. The Company may also make an election to forgo the acquisition of a certain amount of these net operating losses. Since there is a full valuation allowance against the net operating losses, the Company will keep the gross amount of net
F-21
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
operating losses with a full valuation allowance until a future date when it is relevant and the Company can better assess the gross amount of Kintara net operating losses that are available subject to Section 382. Due to the full valuation allowance, a Section 382 Analysis is not relevant at this time.
The Company has not completed an analysis of an ownership change under Section 382 of the Code with respect to TuHURA Delaware. The Company understands due to multiple rounds of financing and debt conversions to equity, there may have been an ownership change under Section 382. The Company plans to perform a Section 382 Analysis in the future when it is relevant. Due to the full valuation allowance, a Section 382 Analysis is not relevant at this time.
In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all the deferred income tax assets will not be realized. The ultimate realization of deferred income tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred income tax liabilities, projected future taxable income, and tax planning strategies in making this assessment. Based on consideration of these items, management has determined that enough uncertainty exists relative to the realization of the deferred income tax asset balances to warrant the application of a full valuation allowance as of December 31, 2024 and 2023.
There was income tax expense for the years ended December 31, 2024 and 2023 of $
Note 12—Stockholders’ equity
Immediately prior to the closing of the Merger, all outstanding shares of Company preferred stock were converted into shares of Company common stock (which were converted into shares of Kintara common stock in the Merger), and upon completion of the merger, all warrants of the Company were converted into warrants to purchase Kintara common stock. All outstanding shares of the Company’s Preferred Stock were converted into
As of December 31, 2024, the Company had two classes of stock defined in its Amended and Restated Articles of Incorporation (the “Articles).
Common Stock – The Company is authorized to issue up to
Preferred Stock – The Company is authorized to issue up to
The historical Kineta Series A Preferred Stock were assumed from the merger and outstanding and have a stated value of $
Warrants –
|
|
Outstanding |
|
|
Weighted average |
|
|
Expiration dates |
||
Historical TuHURA common stock warrants |
|
|
|
|
$ |
|
|
to |
||
Historical Kintara common stock warrants |
|
|
|
|
$ |
|
|
to |
||
2024 common stock warrants issued to financial advisor |
|
|
|
|
$ |
|
|
|||
Warrant modification
In August 2024, the Company extended the exercise period of its common stock purchase warrants issued in connection with Legacy TuHURA Series A Preferred Stock (the “Series A Warrants”) for an additional six months, with a new expiry date of February 12, 2025. There were no other changes in the terms of the Series A Warrants. As a result, a deemed dividend to the holders of the Series A Warrants in the amount of $
F-22
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
the year ended December 31, 2024. The incremental value associated with the warrant modification was determined using a Black-Sholes pricing model using the original terms of the warrants and the modified terms of the warrants and the following assumptions: expected term of approximately
Financial advisor warrants
There were
Warrants exercised
There were
Note 13—Stock option plans
Stock options
The Company uses the Black-Scholes option pricing model to estimate the fair value of stock-based awards on the date of grant. The assumptions employed in the calculation of the fair value of share-based compensation expense were calculated as follows for all periods presented:
|
|
|
2024 |
|
|
2023 |
|
||
Common stock fair value |
|
|
$ |
|
|
$ |
|
||
Risk free interest rate |
|
|
|
|
|
||||
Expected dividend yield |
|
|
|
|
|
||||
Expected term |
|
|
|
|
|
||||
Expected stock volatility |
|
|
|
|
|
||||
Below is a summary of stock option activity for the year ending December 31, 2024:
|
|
|
|
Weighted |
|
|
Weighted |
||
|
Number |
|
|
Average |
|
|
Average |
||
|
of options |
|
|
Exercise Price |
|
|
Contractual Life |
||
Outstanding at December 31, 2023 |
|
|
|
$ |
|
|
|||
Options assumed from Kintara upon merger closing |
|
|
|
$ |
|
|
|||
Forfeited and cancelled |
|
( |
) |
|
$ |
|
|
|
|
Exercised |
|
( |
) |
|
$ |
|
|
|
|
Granted |
|
|
|
$ |
|
|
|
||
Outstanding at December 31, 2024 |
|
|
|
$ |
|
|
|||
Exercisable at December 31, 2024 |
|
|
|
$ |
|
|
|||
Options outstanding had an intrinsic value of $
Restricted Stock units
F-23
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
Restricted stock units “RSU” were assumed from the merger issued to the former Chief Executive Officer and currently is a board of director for the Company and
|
|
|
Number of RSU |
|
|
Restricted stock units assumed from Kintara upon merger closing |
|
|
|
|
|
Vesting of restricted stock units |
|
|
|
|
|
Balance as of December 31, 2024 |
|
|
|
|
|
The remaining RSUs assumed from the Kintara merger will vest on August 1, 2025 and August 1, 2026 and such shares are not accounted for until they vest.
Stock compensation expense
Total stock-based compensation expense was allocated as follows:
|
|
|
2024 |
|
|
2023 |
|
||
General and administrative |
|
|
$ |
|
|
$ |
|
||
Research and development |
|
|
|
|
|
|
|
||
Total stock-based compensation expense |
|
|
$ |
|
|
$ |
|
||
Note 14—Commitments and contingencies
Lease Commitments – The Company leases facilities under non-cancelable operating leases for the laboratory and offices in Tampa, Florida. The current lease expires in February 2026.
Future minimum lease payments under these leases are as follows:
Year ending December 31, 2025 |
|
$ |
|
|
Year ending December 31, 2026 |
|
|
|
|
Interest portion of right of use liability |
|
|
( |
) |
Operating lease liabilities |
|
$ |
|
Total lease expense was approximately $
Cash paid for amounts included in the measurement of lease liabilities was approximately $
For the current lease, the weighted-average lease term is
Employment Agreements – In March 2024, the Company signed a consulting agreement with an entity owned by the former CEO and President and paid approximately $
Future minimum payments under these employment and consulting agreements are as follows:
F-24
TUHURA BIOSCIENSES, INC AND SUBSIDIARIES
Notes to the consolidated financial statements
For the years ended December 31, 2024, and 2023
Year ending December 31, 2025 |
|
$ |
|
|
Year ending December 31, 2026 |
|
|
|
|
|
|
$ |
|
Note 15—Subsequent events
Subsequent events – The Company has evaluated subsequent events through March 31, 2025 in connection with the preparation of these financial statements, which is the date the financial statements were available to be issued.
Payments to Kineta
The Company made payments to Kineta in the amount of $
Warrants exercised
There were
Note receivable to shareholders in connection with warrants exercised
On February 12, 2025, four holders (the “Makers”) of common stock purchase warrants (the “Warrants”) of the Company made and issued to the Company secured promissory notes (the “Warrant Exercise Notes”) in the aggregate principal amount of $
F-25