SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF
THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): January 25, 2013
DELMAR PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
|
Nevada
|
000-54801
|
99-0360497
|
|
(State or Other Jurisdiction of Incorporation)
|
(Commission File Number)
|
(I.R.S. Employer Identification Number)
|
| |
|
|
Suite 720-999 West Broadway
Vancouver, British Columbia
Canada V5Z 1K5
(Address of principal executive offices) (zip code)
(604) 629-5989
(Registrant's telephone number, including area code)
Gregory Sichenzia, Esq.
Jeff Cahlon, Esq.
Sichenzia Ross Friedman Ference LLP
61 Broadway
New York, New York 10006
Phone: (212) 930-9700
Fax: (212) 930-9725
36 Mclean Street, Red Bank, NJ 07701
(Former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
[ ] Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
[ ] Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
[ ] Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
[ ] Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 1.01 Entry into a Material Definitive Agreement.
On January 25, 2013 (the “Closing Date”), DelMar Pharmaceuticals, Inc. (the “Company”) entered into and closed an exchange agreement (the “Exchange Agreement”), with Del Mar Pharmaceuticals (BC) Ltd., a British Columbia corporation (“DelMar (BC)”), 0959454 B.C. Ltd., a British Columbia corporation and a wholly-owned subsidiary of the Company (“Callco”), 0959456 B.C. Ltd., a British Columbia corporation and a wholly-owned subsidiary of the Company (“Exchangeco”), and securityholders of DelMar (BC). Pursuant to the Exchange Agreement, (i) the Company issued 4,340,417 shares of common stock (the “Parent Shares”) to the shareholders of DelMar (BC) who are United States residents (the “U.S. Holders”) in exchange for the transfer to Exchangeco of all 4,340,417 outstanding common shares of DelMar (BC) held by the U.S. Holders, (ii) the shareholders of DelMar (BC) who are Canadian residents (the “Canadian Holders”) received, in exchange for the transfer to Exchangeco of all 8,729,583 outstanding common shares of DelMar (BC) held by the Canadian Holders, 8,729,583 exchangeable shares (the “Exchangeable Shares”) of Exchangeco, and (iii) outstanding warrants to purchase 3,360,000 common shares of DelMar (BC) and outstanding options to purchase 1,020,000 common shares of DelMar (BC) were deemed to be amended such that, rather than entitling the holder to acquire common shares of DelMar (BC), such options and warrants will entitle the holders to acquire shares of common stock of the Company. The Canadian Holders will be entitled to require Exchangeco to redeem (or, at the option of the Company or Callco, to have the Company or Callco purchase) the Exchangeable Shares, and upon such redemption or purchase to receive an equal number of shares of common stock of the Company. The aggregate of 13,070,000 shares of common stock of the Company issued to the Canadian Holders (on an as-exchanged basis with respect to the Exchangeable Shares) and the U.S. Holders represents 80.1% of the outstanding shares of common stock of the Company following the closing of the Exchange Agreement (the “Reverse Acquisition”) and the Share Return (defined below) (not including any shares issuable pursuant to the Private Offering (defined below) or the Valent Agreement Amendment (defined below).
In connection with the Exchange Agreement, on the Closing Date, the Company, Callco and Exchangeco entered into a Support Agreement (the “Support Agreement”). Pursuant to the Support Agreement, the Company agreed that it may not declare or pay any dividend on its common stock unless Exchangeco shall (A) simultaneously declare or pay, as the case may be, an equivalent dividend or other distribution economically equivalent thereto on the Exchangeable Shares (an “Equivalent Dividend”) and take such others actions as are reasonably necessary to ensure that the respective declaration date, record date and payment date for an Equivalent Dividend shall be the same as the declaration date, record date and payment date for the corresponding dividend or other distribution on the Company’s common stock. The Company also agreed to reserve for issuance sufficient authorized shares to allow for the issuance of the Company’s common stock upon the redemption of the Exchangeable Shares, and that it will not, without the prior approval of Exchangeco and the prior approval of the holders of the Exchangeable Shares, issue or distribute (subject to certain exceptions), shares of common stock, rights or options to purchase common stock, or other securities or assets of the Company, to all or substantially of its shareholders unless Exchangeco issues or distributes the economic equivalent of such securities or assets to the holders of the Exchangeable Shares.
In connection with the Exchange Agreement, on the Closing Date, the Company, Callco, Exchangeco and Computershare Trust Company of Canada (the “Trustee”) entered into a voting and exchange trust agreement (the “Trust Agreement”). Pursuant to the Trust Agreement, Company issued one share of Special Voting Preferred Stock (the “Special Voting Share”) to the Trustee, and the parties created a trust for the Trustee to hold the Special Voting Share for the benefit of the holders of the Exchangeable Shares (other than the Company and any affiliated companies) (the “Beneficiaries”). Pursuant to the Trust Agreement, the Beneficiaries will have voting rights in the Company equivalent to what they would have had they received shares of common stock in the same amount as the Exchangeable Shares held by the Beneficiaries.
In connection with the Exchange Agreement and the Trust Agreement, as previously disclosed, on January 17, 2013, the Company filed a certificate of designation of Special Voting Preferred Stock (the “Special Voting Certificate of Designation”) with the Secretary of State of Nevada. Pursuant to the Special Voting Certificate of Designation, one share of the Company’s blank check preferred stock was designated as Special Voting Preferred Stock. The Special Voting Preferred Stock votes as a single class with the common stock and is entitled to a number of votes equal to the number of Exchangeable Shares of Exchangeco outstanding as of the applicable record date (i) that are not owned by the Company or any affiliated companies and (ii) as to which the holder has received voting instructions from the holders of such Exchangeable Shares in accordance with the Trust Agreement.
The Special Voting Preferred Stock is not entitled to receive any dividends or to receive any assets of the Company upon any liquidation, and is not convertible into common stock of the Company.
The voting rights of the Special Voting Preferred Stock will terminate pursuant to and in accordance with the Trust Agreement. The Special Voting Preferred Stock will be automatically cancelled at such time as the share of Special Voting Preferred Stock has no votes attached to it.
In connection with the Exchange Agreement, on the Closing Date, the Company and Exchangeco entered into an intercompany funding agreement (the “Intercompany Funding Agreement”). Pursuant to the Intercompany Funding Agreement, the Company agreed, at the request and on behalf of Exchangeco, to issue the Parent Shares to the U.S. Holders, and Exchangeco agreed to issue to the Company 4,340,417 common shares of Exchangeco.
In connection with the Exchange Agreement, on the Closing Date, the Company entered into and closed a series of subscription agreements with accredited investors (the “Investors”), pursuant to which the Company sold an aggregate of 6,704,938 Units, each Unit consisting of one share of common stock and one five-year warrant (the “Investor Warrants”) to purchase one share of common stock at an exercise price of $0.80, for a purchase price of $0.80 per Unit, for aggregate gross proceeds of $5,363,950 (the “Private Offering”). The exercise price of the Investor Warrants is subject to adjustment in the event that the Company sells common stock at a price lower than the exercise price, subject to certain exceptions. The Investor Warrants are redeemable by the Company at a price of $0.001 per Warrant at any time subject to the conditions that (i) the Company’s common stock has traded for twenty (20) consecutive trading days with a closing price of at least $1.60 per share with an average trading volume of 50,000 shares per day and (ii) the underlying shares of common stock are registered.
The Company retained Charles Vista, LLC (the “Placement Agent”) as the placement agent for the Private Offering and paid the Placement Agent a cash fee of $536,395 (equal to 10% of the gross proceeds), a non-accountable expense allowance of $160,918 (equal to 3% of the gross proceeds), and a consulting fee of $60,000. In addition, the Company issued to the Placement Agent five-year warrants (the “Placement Agent Warrants) to purchase 2,681,975 shares of common stock (equal to 20% of the shares of common stock (i) included as part of the Units sold in the Private Offering and (ii) issuable upon exercise of the Investor Warrants) at an exercise price of $0.80, exercisable on a cash or cashless basis. In addition, the Placement Agent may, for a period of two years from the Closing Date, appoint one person to the Company’s Board of Directors, and one additional person who may attend, as an observer, meetings of the Company’s Board of Directors. The Company has agreed to engage the Placement Agent as its warrant solicitation agent in the event the Investor Warrants are called for redemption and will pay a warrant solicitation fee to the Placement Agent equal to 5% of the amount of funds solicited by the Placement Agent upon the exercise of the Investor Warrants following such redemption.
In connection with the Private Offering, the Company entered into a registration rights agreement with the Investors, pursuant to which the Company agreed to file a registration statement (the “Registration Statement”) registering for resale all shares of common stock (a) included in the Units; and (b) issuable upon exercise of the Investor Warrants, no later than 90 days after the completion of the Private Offering (the “Filing Deadline”) and to use commercially reasonable efforts to cause the Registration Statement to become effective within 180 days of the Filing Deadline. The Company agreed to use commercially reasonably efforts to keep the Registration Statement effective while the Investor Warrants are outstanding.
In connection with the foregoing, the Company relied upon the exemption from securities registration provided by Section 4(2) under the Securities Act of 1933, as amended (the “Securities Act”) for transactions not involving a public offering.
In connection with the Exchange Agreement, in addition to the foregoing:
(i) As previously disclosed, effective January 18, 2013, the Company effected a 3.389831 for 1 stock dividend (“Stock Dividend”) with respect to the outstanding common stock of the Company (such that each stockholder of record as of January 18, 2012 received, as a stock dividend, an additional 2.389831 shares of common stock for each outstanding share of common stock). All share numbers in this report reflect the Stock Dividend unless otherwise indicated.
(ii) As previously closed, effective January 21, 2013, the Company filed Articles of Merger with the Secretary of State of Nevada, pursuant to which the Company’s wholly-owned subsidiary, DelMar Pharmaceuticals, Inc. (formed solely for the purpose of effecting a change in the name of the Company), merged into the Company and the Company changed its name from Berry Only Inc. to DelMar Pharmaceuticals, Inc. In connection with the name change, effective January 30, 2013, the trading symbol of the Company’s common stock changed from “BRRY” to
“DMPI”.
(iii) Effective January 24, 2013, the Company effected a warrant dividend (the “Warrant Dividend”) pursuant to which the Company issued one five-year warrant to purchase one share of common stock at an exercise price of $1.25 for each outstanding share of common stock (the “Dividend Warrants”). Pursuant to the Warrant Dividend, the Company issued an aggregate of 13,369,500 Dividend Warrants.
The Dividend Warrants have the same material terms as the Investor Warrants except as follows:
The exercise price of the Dividend Warrants is $1.25, which will not be adjustable in the event of subsequent equity sales by the Company at a lower price.
The Dividend Warrants will only be exercisable at such times as the underlying shares of common stock are registered.
The Dividend Warrants will be redeemable by the Company at a price of $0.001 per Dividend Warrant at any time commencing 18 months following the date of issuance subject to the conditions that (i) the Company’s common stock has traded for twenty (20) consecutive trading days with a closing price of at least $2.50 per share and (ii) the underlying shares of common stock are registered.
Subject to the conditions set forth therein, the Dividend Warrants may be redeemed by the Company upon not less than sixty (60) days nor more than ninety (90) days prior written notice.
Holders of the Dividend Warrants will have piggyback registration rights with respect to the underlying shares of common stock, subject to the Company’s right to remove any or all of such shares if it determines such removal is necessary or appropriate to ensure such registration statement is declared effective by the Securities and Exchange Commission (the “SEC”) as a result of comments received from the staff of the SEC.
(iv) Effective on the Closing Date, Lisa Guise returned to the Company for cancellation 10,119,493 shares of common stock (the “Share Return”) and 10,119,493 Dividend Warrants.
(v) Effective on the Closing Date (except with respect to Scott Praill, who was appointed on January 29, 2013), Lisa Guise resigned as the sole officer of the Company and the following persons were appointed as executive officers of the Company:
|
Name
|
|
Title
|
|
Jeffrey Bacha
|
|
Chief Executive Officer and President
|
|
Dennis Brown
|
|
Chief Scientific Officer
|
|
Scott Praill
|
|
Chief Financial Officer
|
Effective upon the Company’s meeting its information obligations under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), Lisa Guise will also resign as the sole director of the Company, and Jeffrey Bacha, Dennis Brown, Bill Garner and John K. Bell will be elected directors of the Company.
(vi) Effective on the Closing Date, the Company issued 1,150,000 shares of common stock to Valent Technologies LLC (“Valent”), a company owned by Dennis Brown, the Company’s Chief Scientific Officer, in exchange for Valent agreeing to reduce certain royalties payable to it pursuant to a patent assignment agreement between Valent and DelMar (BC) (the “Valent Agreement Amendment”).
Item 2.01 Completion of Acquisition or Disposition of Assets.
Information in response to this Item 2.01 is keyed to the Item numbers of Form 10.
Item 1. Description of Business.
Effective on the Closing Date, pursuant to the Exchange Agreement, DelMar (BC) became (indirectly through Exchangeco) a wholly-owned subsidiary of the Company. The acquisition of DelMar (BC) is treated as a reverse acquisition, and the business of DelMar (BC) became the business of the Company. At the time of the reverse acquisition, Berry was not engaged in any active business.
References to “we,” “us,” “our” and similar words refer to the Company and its subsidiaries, Callco, Exchangeco and DelMar (BC). References to “Berry” refer to the Company and its business prior to the Reverse Acquisition.
Summary
DelMar (BC) is a British Columbia corporation formed on April 6, 2010. Berry is a Nevada corporation formed on June 24, 2009.
Our executive offices are located at Suite 720-999 West Broadway, Vancouver, British Columbia, Canada V5Z 1K5, and our telephone number at such address is (604) 629-5989.
RISK FACTORS
An investment in the Company’s common stock involves a high degree of risk. In determining whether to purchase the Company’s common stock, an investor should carefully consider all of the material risks described below, together with the other information contained in this report before making a decision to purchase the Company’s securities. An investor should only purchase the Company’s securities if he or she can afford to suffer the loss of his or her entire investment.
Risks related to our Business and our Industry
We have a limited operating history and a history of operating losses, and expect to incur significant additional operating losses.
We are a development stage company. DelMar (BC) was formed in British Columbia in 2010 and has only a limited operating history. Therefore, there is limited historical financial information upon which to base an evaluation of our performance. Our prospects must be considered in light of the uncertainties, risks, expenses, and difficulties frequently encountered by companies in their early stages of operations. We have generated net losses since we began operations, including $1,333,011 for year ended December 31, 2011 and $796,947 for the nine months ended September 30, 2012. We expect to incur substantial additional net expenses over the next several years as our research, development, and commercial activities increase. The amount of future losses and when, if ever, we will achieve profitability are uncertain. Our ability to generate revenue and achieve profitability will depend on, among other things, successful completion of the preclinical and clinical development of our product candidates; obtaining necessary regulatory approvals from the U.S. Food and Drug Administration (the “FDA”) and international regulatory agencies; successful manufacturing, sales, and marketing arrangements; and raising sufficient funds to finance our activities. If we are unsuccessful at some or all of these undertakings, our business, prospects, and results of operations may be materially adversely affected.
We may need to secure additional financing.
We anticipate that we will incur operating losses for the foreseeable future. We may require additional funds for our anticipated operations and if we are not successful in securing additional financing, we may be required to delay significantly, reduce the scope of or eliminate one or more of our research or development programs, downsize our general and administrative infrastructure, or seek alternative measures to avoid insolvency, including arrangements with collaborative partners or others that may require us to relinquish rights to certain of our technologies, product candidates or products.
Our auditors have issued a “going concern” audit opinion.
Our independent auditors have indicated, in their report on our December 31, 2011 financial statements, that there is substantial doubt about our ability to continue as a going concern. A “going concern” opinion indicates that the financial statements have been prepared assuming we will continue as a going concern and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets, or the amounts and classification of liabilities that may result if we do not continue as a going concern. Therefore, you should not rely on our balance sheet as an indication of the amount of proceeds that would be available to satisfy claims of creditors, and potentially be available for distribution to shareholders, in the event of liquidation.
We are an early-stage company with an unproven business strategy and may never achieve commercialization of our candidate products or profitability.
We are at an early stage of development and commercialization of our technologies and product candidates. We have not yet begun to market any products and, accordingly, have not begun or generate revenues from the commercialization of our products. Our products will require significant additional clinical testing and investment prior to commercialization. A commitment of substantial resources by ourselves and, potentially, our partners to conduct time-consuming research and clinical trials will be required if we are to complete the development of our product candidates. There can be no assurance that any of our product candidates will meet applicable regulatory standards, obtain required regulatory approvals, be capable of being produced in commercial quantities at reasonable costs or be successfully marketed. Most of our product candidates are not expected to be commercially available for several years, if at all.
Our and our collaborators ability to sell therapeutic products will depend to a large extent upon reimbursement from health care insurance companies.
Our success may depend, in part, on the extent to which reimbursement for the costs of therapeutic products and related treatments will be available from third-party payers such as government health administration authorities, private health insurers, managed care programs, and other organizations. Over the past decade, the cost of health care has risen significantly, and there have been numerous proposals by legislators, regulators, and third-party health care payers to curb these costs. Some of these proposals have involved limitations on the amount of reimbursement for certain products. Similar federal or state health care legislation may be adopted in the future and any products that we or our collaborators seek to commercialize may not be considered cost-effective. Adequate third-party insurance coverage may not be available for us or our collaborative partners to establish and maintain price levels that are sufficient for realization of an appropriate return on investment in product development.
We are dependent on obtaining certain patents and protecting our proprietary rights.
Our success will depend, in part, on our ability to obtain patents, maintain trade secret protection and operate without infringing on the proprietary rights of third parties or having third parties circumvent our rights. We have filed and are actively pursuing patent applications for our products. The patent positions of biotechnology, biopharmaceutical and pharmaceutical companies can be highly uncertain and involve complex legal and factual questions. Thus, there can be no assurance that any of our patent applications will result in the issuance of patents, that we will develop additional proprietary products that are patentable, that any patents issued to us or those that already have been issued will provide us with any competitive advantages or will not be challenged by any third parties, that the patents of others will not impede our ability to do business or that third parties will not be able to circumvent our patents. Furthermore, there can be no assurance that others will not independently develop similar products, duplicate any of our products not under patent protection, or, if patents are issued to us, design around the patented products we developed or will develop.
We may be required to obtain licenses from third parties to avoid infringing patents or other proprietary rights. No assurance can be given that any licenses required under any such patents or proprietary rights would be made available, if at all, on terms we find acceptable. If we do not obtain such licenses, we could encounter delays in the introduction of products, or could find that the development, manufacture or sale of products requiring such licenses could be prohibited.
A number of pharmaceutical, biopharmaceutical and biotechnology companies and research and academic institutions have developed technologies, filed patent applications or received patents on various technologies that may be related to or affect our business. Some of these technologies, applications or patents may conflict with our technologies or patent applications. Such conflict could limit the scope of the patents, if any, that we may be able to obtain or result in the denial of our patent applications. In addition, if patents that cover our activities are issued to other companies, there can be no assurance that we would be able to obtain licenses to these patents at a reasonable cost or be able to develop or obtain alternative technology. If we do not obtain such licenses, we could encounter delays in the introduction of products, or could find that the development, manufacture or sale of products requiring such licenses could be prohibited. In addition, we could incur substantial costs in defending ourselves in suits brought against us on patents it might infringe or in filing suits against others to have such patents declared invalid.
Patent applications in the U.S. are maintained in secrecy and not published if either: i) the application is a provisional application or, ii) the application is filed and we request no publication, and certify that the invention disclosed “has not and will not” be the subject of a published foreign application. Otherwise, U.S. applications or foreign counterparts, if any, publish 18 months after the priority application has been filed. Since publication of discoveries in the scientific or patent literature often lag behind actual discoveries, we cannot be certain that we or any licensor were the first creator of inventions covered by pending patent applications or that we or such licensor was the first to file patent applications for such inventions. Moreover, we might have to participate in interference proceedings declared by the U.S. Patent and Trademark Office to determine priority of invention, which could result in substantial cost to us, even if the eventual outcome were favorable to us. There can be no assurance that our patents, if issued, would be held valid or enforceable by a court or that a competitor’s technology or product would be found to infringe such patents.
Much of our know-how and technology may not be patentable. To protect our rights, we require employees, consultants, advisors and collaborators to enter into confidentiality agreements. There can be no assurance, however, that these agreements will provide meaningful protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure. Further, our business may be adversely affected by competitors who independently develop competing technologies, especially if we obtain no, or only narrow, patent protection.
We are subject to various government regulations.
The manufacture and sale of human therapeutic and diagnostic products in the U.S., Canada and foreign jurisdictions are governed by a variety of statutes and regulations. These laws require approval of manufacturing facilities, controlled research and testing of products and government review and approval of a submission containing manufacturing, preclinical and clinical data in order to obtain marketing approval based on establishing the safety and efficacy of the product for each use sought, including adherence to current Good Manufacturing Practice (or cGMP) during production and storage, and control of marketing activities, including advertising and labeling.
The products we are currently developing will require significant development, preclinical and clinical testing and investment of substantial funds prior to their commercialization. The process of obtaining required approvals can be costly and time-consuming, and there can be no assurance that future products will be successfully developed and will prove to be safe and effective in clinical trials or receive applicable regulatory approvals. Markets other than the U.S. and Canada have similar restrictions. Potential investors and shareholders should be aware of the risks, problems, delays, expenses and difficulties which we may encounter in view of the extensive regulatory environment which controls our business.
If we are unable to keep up with rapid technological changes in our field or compete effectively, we will be unable to operate profitably.
We are engaged in a rapidly changing field. Other products and therapies that will compete directly with the products that we are seeking to develop and market currently exist or are being developed. Competition from fully integrated pharmaceutical companies and more established biotechnology companies is intense and is expected to increase. Most of these companies have significantly greater financial resources and expertise in discovery and development, manufacturing, preclinical and clinical testing, obtaining regulatory approvals and marketing than us. Smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large pharmaceutical and established biopharmaceutical or biotechnology companies. Many of these competitors have significant products that have been approved or are in development and operate large, well-funded discovery and development programs. Academic institutions, governmental agencies and other public and private research organizations also conduct research, seek patent protection and establish collaborative arrangements for therapeutic products and clinical development and marketing. These companies and institutions compete with us in recruiting and retaining highly qualified scientific and management personnel. In addition to the above factors, we will face competition based on product efficacy and safety, the timing and scope of regulatory approvals, availability of supply, marketing and sales capability, reimbursement coverage, price and patent position. There is no assurance that our competitors will not develop more effective or more affordable products, or achieve earlier patent protection or product commercialization, than our own.
Other companies may succeed in developing products earlier than ourselves, obtaining Health Canada, European Medicines Agency (the “EMEA”) and FDA approvals for such products more rapidly than we will, or in developing products that are more effective than products we propose to develop. While we will seek to expand our technological capabilities in order to remain competitive, there can be no assurance that research and development by others will not render our technology or products obsolete or non-competitive or result in treatments or cures superior to any therapy we develop, or that any therapy we develop will be preferred to any existing or newly developed technologies.
Clinical trials for our product candidates are expensive and time consuming, and their outcome is uncertain.
The process of obtaining and maintaining regulatory approvals for new therapeutic products is expensive, lengthy and uncertain. Costs and timing of clinical trials may vary significantly over the life of a project owing to any or all of the following non-exclusive reasons:
|
●
|
the duration of the clinical trial;
|
|
●
|
the number of sites included in the trials;
|
|
●
|
the countries in which the trial is conducted;
|
|
●
|
the length of time required and ability to enroll eligible patients;
|
|
●
|
the number of patients that participate in the trials;
|
|
●
|
the number of doses that patients receive;
|
|
●
|
the drop-out or discontinuation rates of patients;
|
|
●
|
per patient trial costs;
|
|
●
|
third party contractors failing to comply with regulatory requirements or meet their contractual obligations to us in a timely manner;
|
|
●
|
our final product candidates having different properties in humans than in laboratory testing;
|
|
●
|
the need to suspect or terminate our clinical trials;
|
|
●
|
insufficient or inadequate supply of quality of necessary materials to conduct our trials;
|
|
●
|
potential additional safety monitoring, or other conditions required by FDA or comparable foreign regulatory authorities regarding the scope or design of our clinical trials, or other studies requested by regulatory agencies;
|
|
●
|
problems engaging institutional review boards, or IRBs, to oversee trials or in obtaining and maintaining IRB approval of studies;
|
|
●
|
the duration of patient follow-up;
|
|
●
|
the efficacy and safety profile of a product candidate;
|
|
●
|
the costs and timing of obtaining regulatory approvals; and
|
|
●
|
the costs involved in enforcing or defending patent claims or other intellectual property rights.
|
Late stage clinical trials are especially expensive, typically requiring tens of millions of dollars, and take years to reach their outcomes. Such outcomes often fail to reproduce the results of earlier trials. It is often necessary to conduct multiple late stage trials (including multiple Phase III trials) in order to obtain sufficient results to support product approval, which further increases the expense. Sometimes trials are further complicated by changes in requirements while the trials are under way (for example, when the standard of care changes for the disease that is being studied in the trial). Accordingly, any of our current or future product candidates could take a significantly longer time to gain regulatory approval than we expect, or may never gain approval, either of which could delay or stop the commercialization of our product candidates.
We may be required to suspend or discontinue clinical trials due to unexpected side effects or other safety risks that could preclude approval of our product candidates.
Our clinical trials may be suspended at any time for a number of reasons. For example, we may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to the clinical trial patients. In addition, the FDA or other regulatory agencies may order the temporary or permanent discontinuation of our clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to the clinical trial patients.
Administering any product candidate to humans may produce undesirable side effects. These side effects could interrupt, delay or halt clinical trials of our product candidates and could result in the FDA or other regulatory authorities denying further development or approval of our product candidates for any or all targeted indications. Ultimately, some or all of our product candidates may prove to be unsafe for human use. Moreover, we could be subject to significant liability if any volunteer or patient suffers, or appears to suffer, adverse health effects as a result of participating in our clinical trials.
We may not receive regulatory approvals for our product candidates or there may be a delay in obtaining such approvals.
Our products and our ongoing development activities are subject to regulation by regulatory authorities in the countries in which we or our collaborators and distributors wish to test, manufacture or market our products. For instance, the FDA will regulate the product in the U.S. and equivalent authorities, such as the European Medicines Agency, or EMA, will regulate in Europe. Regulatory approval by these authorities will be subject to the evaluation of data relating to the quality, efficacy and safety of the product for its proposed use, and there can be no assurance that the regulatory authorities will find our data sufficient to support product approval of VAL-083.
The time required to obtain regulatory approval varies between countries. In the U.S., for products without “Fast Track” status, it can take up to eighteen (18) months after submission of an application for product approval to receive the FDA's decision. Even with Fast Track status, FDA review and decision can take up to twelve (12) months. At present, we do not have Fast Track status for our lead product candidate, VAL-083.
Different regulators may impose their own requirements and may refuse to grant, or may require additional data before granting, an approval, notwithstanding that regulatory approval may have been granted by other regulators. Regulatory approval may be delayed, limited or denied for a number of reasons, including insufficient clinical data, the product not meeting safety or efficacy requirements or any relevant manufacturing processes or facilities not meeting applicable requirements as well as case load at the regulatory agency at the time.
We may fail to comply with regulatory requirements.
Our success will be dependent upon our ability, and our collaborative partners’ abilities, to maintain compliance with regulatory requirements, including cGMP, and safety reporting obligations. The failure to comply with applicable regulatory requirements can result in, among other things, fines, injunctions, civil penalties, total or partial suspension of regulatory approvals, refusal to approve pending applications, recalls or seizures of products, operating and production restrictions and criminal prosecutions.
Regulatory approval of our product candidates may be withdrawn at any time.
After regulatory approval has been obtained for medicinal products, the product and the manufacturer are subject to continual review, including the review of adverse experiences and clinical results that are reported after our products are made available to patients, and there can be no assurance that such approval will not be withdrawn or restricted. Regulators may also subject approvals to restrictions or conditions, or impose post-approval obligations on the holders of these approvals, and the regulatory status of such products may be jeopardized if such obligations are not fulfilled. If post-approval studies are required, such studies may involve significant time and expense.
The manufacturer and manufacturing facilities we use to make any of our products will also be subject to periodic review and inspection by the FDA or EMA, as applicable. The discovery of any new or previously unknown problems with the product, manufacturer or facility may result in restrictions on the product or manufacturer or facility, including withdrawal of the product from the market. We will continue to be subject to the FDA or EMA requirements, as applicable, governing the labeling, packaging, storage, advertising, promotion, recordkeeping, and submission of safety and other post-market information for all of our product candidates, even those that the FDA or EMA, as applicable, had approved. If we fail to comply with applicable continuing regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory approval, product recalls and seizures, operating restrictions and other adverse consequences.
There may not be a viable market for our products.
We believe that there will be many different applications for our products. We also believe that the anticipated market for our products will continue to expand. These assumptions may prove to be incorrect for a variety of reasons, including competition from other products and the degree of our products’ commercial viability.
We rely on key personnel and, if we are unable to retain or motivate key personnel or hire qualified personnel, we may not be able to grow effectively.
We are dependent on certain members of our management, scientific and drug development staff and consultants, the loss of services of one or more of whom could materially adversely affect us.
We currently do not have full-time employees, but retain the services of approximately 19 persons on an independent contractor/consultant and contract-employment basis. Our ability to manage growth effectively will require us to continue to implement and improve our management systems and to recruit and train new employees. Although we have done so in the past and expect to do so in the future, there can be no assurance that we will be able to successfully attract and retain skilled and experienced personnel.
We may be subject to foreign exchange fluctuation.
We maintain our accounts in Canadian dollars. A portion of our expenditures are in foreign currencies, most notably in U.S. dollars, and therefore we are subject to foreign currency fluctuations, which may, from time to time, impact our financial position and results. We may enter into hedging arrangements under specific circumstances, typically through the use of forward or futures currency contracts, to minimize the impact of increases in the value of the U.S. dollar. In order to minimize our exposure to foreign exchange fluctuations we may hold sufficient U.S. dollars to cover our expected U.S. dollar expenditures.
We may be exposed to potential product and clinical trials liability.
Our business exposes us to potential product liability risks, which are inherent in the testing, manufacturing, marketing and sale of therapeutic products. Human therapeutic products involve an inherent risk of product liability claims and associated adverse publicity. While we will continue to take precautions we deem appropriate, there can be no assurance that we will be able to avoid significant product liability exposure. We maintain liability insurance coverage. Such insurance is expensive, difficult to obtain and may not continue to be available on acceptable terms, if at all. An inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of our current or potential products. A product liability claim brought against us in a clinical trial or a product withdrawal could have a material adverse effect upon us and our financial condition.
We are dependent on our collaborative partners and service providers the loss of which would hurt our business.
Our strategy is to enter into various arrangements with corporate and academic collaborators, licensors, licensees, service providers and others for the research, development, clinical testing and commercialization of our products. We intend to or have entered into agreements with academic, medical and commercial organizations to research, develop and test our products. In addition, we intend to enter into corporate partnerships to commercialize the Company’s core products. There can be no assurance that such collaborations can be established on favorable terms, if at all.
Should any collaborative partner or service provider fail to appropriately research, develop, test or successfully commercialize any product to which the Company has rights, our business may be adversely affected. Failure of a collaborative partner or service provider to successfully conduct or complete their activities or to remain a viable collaborative partner or commercialize enterprise for any particular program could delay or halt the development or commercialization of any products arising out of such program. While management believes that collaborative partners and service providers will have sufficient economic motivation to continue their activities, there can be no assurance that any of these collaborations or provisions of required services will be continued or result in successfully commercialized products.
In addition, there can be no assurance that the collaborative research or commercialization partners will not pursue alternative technologies or develop alternative products either on their own or in collaboration with others, including our competitors, as a means for developing treatments for the diseases or conditions targeted by our programs.
We may become subject to liabilities related to risks inherent in working with hazardous materials.
Our discovery and development processes involve the controlled use of hazardous and radioactive materials. We are subject to federal, provincial and local laws and regulations governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by such laws and regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result and any such liability could exceed our resources. We are not specifically insured with respect to this liability. Although we believe that we are in compliance in all material respects with applicable environmental laws and regulations and currently do not expect to make material capital expenditures for environmental control facilities in the near-term, there can be no assurance that we will not be required to incur significant costs to comply with environmental laws and regulations in the future, or that our operations, business or assets will not be materially adversely affected by current or future environmental laws or regulations.
Risks Related to Our Common Stock
There is not an active liquid trading market for the Company’s common stock.
The Company files reports under the Exchange Act and its common stock is eligible for quotation on the OTC Bulletin Board. However, there is no regular active trading market in the Company’s common stock, and we cannot give an assurance that an active trading market will develop. If an active market for the Company’s common stock develops, there is a significant risk that the Company’s stock price may fluctuate dramatically in the future in response to any of the following factors, some of which are beyond our control:
| ● |
variations in our quarterly operating results; |
| |
|
| ● |
announcements that our revenue or income are below analysts’ expectations; |
| |
|
| ● |
general economic slowdowns; |
| |
|
| ● |
sales of large blocks of the Company’s common stock; and |
| |
|
| ● |
announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments. |
Our common stock is subject to the “penny stock” rules of the Securities and Exchange Commission, which may make it more difficult for stockholders to sell our common stock.
The SEC has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require that a broker or dealer approve a person’s account for transactions in penny stocks, and the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must obtain financial information and investment experience objectives of the person, and make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form sets forth the basis on which the broker or dealer made the suitability determination, and that the broker or dealer received a signed, written agreement from the investor prior to the transaction.
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of the Company’s common stock if and when such shares are eligible for sale and may cause a decline in the market value of its stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stock.
Because we became a public by means of a reverse acquisition, we may not be able to attract the attention of brokerage firms.
Because we became public through a “reverse acquisition”, securities analysts of brokerage firms may not provide coverage of us since there is little incentive to brokerage firms to recommend the purchase of our common stock. No assurance can be given that brokerage firms will want to conduct any secondary offerings on behalf of the Company in the future.
Applicable regulatory requirements, including those contained in and issued under the Sarbanes-Oxley Act of 2002, may make it difficult for the Company to retain or attract qualified officers and directors, which could adversely affect the management of its business and its ability to obtain or retain listing of its common stock.
The Company may be unable to attract and retain those qualified officers, directors and members of board committees required to provide for effective management because of the rules and regulations that govern publicly held companies, including, but not limited to, certifications by principal executive officers. The enactment of the Sarbanes-Oxley Act has resulted in the issuance of a series of related rules and regulations and the strengthening of existing rules and regulations by the SEC, as well as the adoption of new and more stringent rules by the stock exchanges. The perceived increased personal risk associated with these changes may deter qualified individuals from accepting roles as directors and executive officers.
Further, some of these changes heighten the requirements for board or committee membership, particularly with respect to an individual’s independence from the corporation and level of experience in finance and accounting matters. The Company may have difficulty attracting and retaining directors with the requisite qualifications. If the Company is unable to attract and retain qualified officers and directors, the management of its business and its ability to obtain or retain listing of our shares of common stock on any stock exchange (assuming the Company elects to seek and are successful in obtaining such listing) could be adversely affected.
If the Company fails to maintain an effective system of internal controls, it may not be able to accurately report its financial results or detect fraud. Consequently, investors could lose confidence in the Company’s financial reporting and this may decrease the trading price of its stock.
The Company must maintain effective internal controls to provide reliable financial reports and detect fraud. The Company has been assessing its internal controls to identify areas that need improvement. It is in the process of implementing changes to internal controls, but has not yet completed implementing these changes. Failure to implement these changes to the Company’s internal controls or any others that it identifies as necessary to maintain an effective system of internal controls could harm its operating results and cause investors to lose confidence in the Company’s reported financial information. Any such loss of confidence would have a negative effect on the trading price of the Company’s stock.
Voting power of our shareholders is highly concentrated by insiders.
he Company’s officers and directors beneficially own approximately 47% of our outstanding shares of common stock. Such concentrated control of the Company may adversely affect the price of our common stock. If you acquire common stock, you may have no effective voice in the management of the Company. Sales by insiders or affiliates of the Company, along with any other market transactions, could affect the market price of our common stock.
We do not intend to pay dividends for the foreseeable future.
We have paid no dividends on our common stock to date and it is not anticipated that any dividends will be paid to holders of our common stock in the foreseeable future. While our future dividend policy will be based on the operating results and capital needs of the business, it is currently anticipated that any earnings will be retained to finance our future expansion and for the implementation of our business plan. As an investor, you should take note of the fact that a lack of a dividend can further affect the market value of our stock, and could significantly affect the value of any investment in our Company.
Our articles of incorporation allow for our board to create new series of preferred stock without further approval by our stockholders, which could adversely affect the rights of the holders of our Common Stock.
Our Board of Directors has the authority to fix and determine the relative rights and preferences of preferred stock. Our Board of Directors have the authority to issue up to 5,000,000 shares of our preferred stock (of which 1 share has been designated Special Voting Preferred Stock and is issued and outstanding) without further stockholder approval. As a result, our Board of Directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock and the right to the redemption of the shares, together with a premium, prior to the redemption of our common stock. In addition, our board of directors could authorize the issuance of a series of preferred stock that has greater voting power than our common stock or that is convertible into our common stock, which could decrease the relative voting power of our common stock or result in dilution to our existing stockholders. Although we have no present intention to issue any additional shares of preferred stock or to create any additional series of preferred stock, we may issue such shares in the future.
If and when a registration statement becomes effective, there will be a significant number of shares of common stock eligible for sale, which could depress the market price of such shares.
We have agreed to file a registration statement with the SEC to register the shares of our common stock issued or issuable in connection with the Private Offering. Following the effective date of such registration statement a large number of shares of common stock will be available for sale in the public market, which could harm the market price of the stock.
As an issuer of “penny stock”, the protection provided by the federal securities laws relating to forward looking statements does not apply to us.
Although federal securities laws provide a safe harbor for forward-looking statements made by a public company that files reports under the federal securities laws, this safe harbor is not available to issuers of penny stocks. As a result, we will not have the benefit of this safe harbor protection in the event of any legal action based upon a claim that the material provided by us contained a material misstatement of fact or was misleading in any material respect because of our failure to include any statements necessary to make the statements not misleading. Such an action could hurt our financial condition.
Our issuance of common stock upon exercise of warrants or options may depress the price of our common stock.
As of January 30, 2013, we have 15,445,362 shares of common stock, 8,729,583 shares of common stock issuable upon exchange of the Exchangeable Shares, warrants to purchase 15,996,920 shares of common stock, and options to purchase 1,020,000 shares of common stock, issued and outstanding. The issuance of shares of common stock upon exercise of outstanding warrants or options could result in substantial dilution to our stockholders, which may have a negative effect on the price of our common stock.
FORWARD-LOOKING STATEMENTS
Statements in this current report on Form 8-K may be “forward-looking statements.” Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates and projections about our business based, in part, on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Therefore, actual outcomes and results may, and are likely to, differ materially from what is expressed or forecasted in the forward-looking statements due to numerous factors, including those described above and those risks discussed from time to time in this report, including the risks described under “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this report and in other documents which we file with the Securities and Exchange Commission. In addition, such statements could be affected by risks and uncertainties related to:
|
●
|
our ability to raise funds for general corporate purposes and operations, including our clinical trials;
|
|
●
|
the commercial feasibility and success of our technology;
|
|
●
|
our ability to recruit qualified management and technical personnel;
|
|
●
|
the success of our clinical trials;
|
|
●
|
our ability to obtain and maintain required regulatory approvals for our products; and
|
|
●
|
the other factors discussed in the “Risk Factors” section and elsewhere in this report.
|
Any forward-looking statements speak only as of the date on which they are made, and except as may be required under applicable securities laws, we do not undertake any obligation to update any forward-looking statement to reflect events or circumstances after the date of this current report.
Business
Berry is a Nevada corporation formed on June 24, 2009. On July 8, 2010, Berry entered into an exclusive dealership agreement with Wireless Wipes, a New York corporation that manufactures a sanitizing wipe used to clean cell phones and other mobile devices. The agreement granted Berry the exclusive right to purchase, inventory, promote and resell the product within Canada under certain minimum order rules. The agreement required an annual distribution of 10,000 pouches of product. Berry was unable to generate the required annual sales and the agreement lapsed. Prior to the Reverse Acquisition, Berry did not have any significant assets or operations.
Del Mar Pharmaceuticals (BC) Ltd. is a British Columbia, Canada corporation founded in 2010. We are a clinical and commercial stage drug development company with a focus on the treatment of cancer. Our mission is to benefit patients and create shareholder value by rapidly developing and commercializing anti-cancer therapies in orphan cancer indications where patients have failed modern therapy.
Our lead product candidate, VAL-083, represents a “first in class” small-molecule chemotherapeutic, which means that the molecular structure of VAL-083 is not an analogue or derivative of other small molecule chemotherapeutics approved for the treatment of cancer. VAL-083 has been assessed in multiple clinical studies sponsored by the National Cancer Institute (NCI) in the United States as a treatment against various cancers including lung, brain, cervical, ovarian tumors and leukemia. Published pre-clinical and clinical data suggest that VAL-083 may be active against a range of tumor types. VAL-083 is approved as a cancer chemotherapeutic in China for the treatment of chronic myelogenous leukemia and lung cancer. VAL-083 has not been approved for any indication outside of China.
Upon obtaining regulatory approval, we intend to commercialize VAL-083 and other product candidates for the treatment of orphan and other cancer indications where patients have failed other therapies or have limited medical options. Orphan diseases are defined in the United States under the Rare Disease Act of 2002 as “any disease or condition that affects less than 200,000 persons in the United States”. The Orphan Drug Act of 1983 is a federal law that provides financial and other incentives including a period of market exclusivity to encourage the development of new treatments for orphan diseases. In February 2012, we announced that VAL-083 has been granted protection under the Orphan Drug Act by the United States Food and Drug Administration for the treatment of glioma, including glioblastoma multiforme (“GBM).
We research the mechanism of action of our product candidates to determine the clinical indications best suited for therapy and rapidly advance our product candidates into human clinical trials and toward commercialization.
With this aim, we have initiated clinical trials with VAL-083 as a potential new treatment for GBM, the most common and aggressive form of brain cancer. In April 2012, we presented data at the American Association of Cancer Research (AACR website: http://www.aacr.org) annual meeting demonstrating that VAL-083 maintains activity in tumors resistant to the current front-line GBM therapy, Temodar®. In November 2012, we presented interim data from our clinical trial at the Annual Meeting of the Society for NeuroOncology (SNO website: http://www.soc-neuro-onc.org) demonstrating that VAL-083 can shrink or halt the growth of tumors in brain cancer patients who have failed other approved treatments. Currently, there is no approved therapy for these patients.
In addition to our clinical development activities in the United States, we have obtained exclusive commercial rights to VAL-083 in China. In October 2012, we announced that we had entered into a collaboration agreement with the only manufacturer presently licensed by the Chinese State Food and Drug Administration (SFDA) to produce the product for the China market. This agreement provides us with exclusive commercial rights, which positions us to generate near-term revenue through product sales or royalties for its approved indications in China while we seek global approval in new indications. We anticipate that we may be able to begin generating revenue from such sales or royalties commencing in 2013.
VAL-083 was originally discovered in the 1960’s. We have a broad portfolio of new patent applications to protect our intellectual property. Our patent applications claim compositions and methods related to the use of VAL-083 and related compounds as well as methods of synthesis and quality controls for the manufacturing process of VAL-083. In addition, VAL-083 has been granted protection under the Orphan Drug Act by the United States Food and Drug Administration (USA). We believe that our portfolio of intellectual property rights provides a strong and defensible market position for the commercialization of VAL-083 and other anti-cancer products.
We also believe the experience of our clinical development team will position us to acquire or license additional product candidates to establish a pipeline of product opportunities. We have secured three grants from the National Research Council of Canada, which have provided financial contributions of over Cdn $130,000 to date. We believe we have the potential to create significant value by building and maintaining a sustainable business through the commercialization of VAL-083 and other products across a variety of cancer indications on a world-wide basis.
The Technology
Our drug discovery research focuses on identifying well-validated clinical and commercial-stage compounds and establishing a scientific rationale for development in modern orphan drug indications. Through our relationship with Valent, a company owned by Dr. Dennis Brown, our chief scientific officer, we are able to utilize Valent’s proprietary ChemState™ bioinformatics tools which is used to screen and identify potential candidates. Promising candidates are further researched through our network of expert consultants and contract research organizations. This approach allows us to rapidly identify and advance potential drug candidates without significant investment in “wet lab” infrastructure. Based on this strategy, we acquired initial VAL-083 intellectual property and prototype drug product from Valent and have identified multiple additional drug candidates that we may have the opportunity to license or acquire in the future.
VAL-083
VAL-083 is a novel “first in class” small-molecule therapeutic agent that we are developing as a new cancer chemotherapy.
VAL-083 has been assessed in multiple NCI-sponsored clinical studies in various cancers including lung, brain, cervical, ovarian tumors and leukemia. Published pre-clinical and clinical data from the late 1970s and 1980s suggest that VAL-083 may be active against a range of tumor types; however, further research was not pursued in the United States due to an increased focus by the NCI on targeted biologic therapies during the era. VAL-083 is approved as a cancer chemotherapeutic in China for the treatment of chronic myelogenous (or myeloid) leukemia (“CML”) and lung cancer.
The mechanism of action of VAL-083 is understood to be a bi-functional alkylating agent. Alkylating agents are a commonly used class of chemotherapy drugs. They work by binding to DNA and interfering with normal processes within the cancer cell, which prevents the cell from making the proteins needed to grow and survive. After exposure to alkylating agents, the cancer cell becomes dysfunctional and dies. There are a number of alkylating agents on the market that are used by physicians to treat different types of cancer.
Based on published research, the functional groups associated with the mechanism of action of VAL-083 are understood to be functionally different from commonly used alkylating agents, including Temodar®, which is commonly used a front-line chemotherapy against GBM, the most common and aggressive form of brain cancer. VAL-083 has previously demonstrated activity in cell-lines that are resistant to other types of chemotherapy. No evidence of cross-resistance has been reported in published clinical studies. Based on the presumed alkylating functionality of VAL-083, published literature suggests that DNA repair mechanisms associated with the leading brain cancer therapies, including Temodar® and nitrosourea resistance may not confer resistance to VAL-083. Therefore, we believe that VAL-083 may be effective in treating tumors that have failed or become resistant to other chemotherapies.
We have presented new research at the American Association of Cancer Research (AACR) demonstrating that VAL-083 is active in patient-derived tumor cell lines and cancer stem cells that are resistant to other chemotherapies. Of particular importance is resistance to Temodar® due to activity of the repair enzyme known as MGMT, which results in resistance to front-line therapy in many GBM patients. At AACR, we presented data demonstrating that VAL-083 is active independent of MGMT resistance in laboratory studies
VAL-083 readily crosses the blood brain barrier where it maintains a long half-life in comparison to the plasma. Published preclinical and clinical research demonstrates that VAL-083 is selective for brain tumor tissue.
VAL-083 has been assessed in multiple studies as chemotherapy in the treatment of newly diagnosed and recurrent brain tumors and other cancers. In general, tumor regression in brain cancer was achieved following therapy in greater than 40% of patients treated and stabilization was achieved in an additional 20% - 30%. In published clinical studies, VAL-083 has previously been shown to have a statistically significant impact on median survival in high grade glioma brain tumors when combined with radiation vs. radiation alone.
A summary of published data adapted from separate sources comparing the efficacy of VAL-083 and other therapies
in the treatment of glioblastoma multiforme (GBM).
The main dose-limiting toxicity related to the administration of VAL-083 in previous NCI-sponsored clinical studies was myelosuppression. Myelosuppression is the decrease in cells responsible for providing immunity, carrying oxygen, and those responsible for normal blood clotting. Bone marrow suppression is a common side effect of chemotherapy There is no evidence of lung, liver or kidney toxicity even with prolonged treatment by VAL-083. Commercial data from the Chinese market where the drug has been approved for more than 15 years supports the safety findings of the NCI studies.
We note that the dose-limiting toxicity of VAL-083 was established prior to the development of medicines now available to manage myelosuppression. Various types of medications and other forms of therapy are now available for management of myelosuppressive side effects. We believe this offers the potential of increasing the dose of VAL-083 in the modern patient population thereby providing a potential opportunity to improve the drug’s already established efficacy profile.
VAL-083 Clinical Development in GBM
Based on historical data and our own research, we filed an investigational new drug (IND) application with the FDA and initiated human clinical trials with VAL-083 as a potential treatment for GBM in 2011.
Our clinical trial is a Phase I/II an open-label, single arm dose-escalation study designed to evaluate the safety, tolerability, pharmacokinetics and anti-cancer activity of VAL-083 in patients with GBM. To be eligible for our clinical trial, patients must have been previously treated for GBM with surgery and/or radiation, if appropriate, and must have failed both Bevacizumab (Avastin®) and temozolomide (Temodar®), unless either or both are contra-indicated. Patients with brain tumors that have developed due to CNS metastases are also eligible for the study.
Response to treatment with VAL-083 is measured prior to each treatment cycle. An initial phase of the study involves dose escalation cohorts until a maximum tolerated dose (MTD) is established in the context of modern care. Once the modernized dosing regimen has been established, additional patients will be enrolled at the MTD (or other selected optimum dosing regimen) in a registration directed Phase II clinical trial.
In February 2012, we announced that VAL-083 has been granted protection under the Orphan Drug Act by the FDA. Based on historical development of other products in GBM, we believe that we may be able to obtain FDA approval from an open-label Phase II registration-directed clinical, which will save significant costs of a large Phase III clinical trial. We also believe that the FDA may grant fast-track, accelerated approval and/or priority review status to VAL-083, which will enable us to begin filing for commercial approval during the clinical trial process. Fast Track, Accelerated Approval and Priority Review are approaches established by the FDA that are intended to make therapeutically important drugs available at an earlier time. (See “Government Regulation and Product Approval”.)
We are conducting the study under the direction of Dr. Howard Burris at the Sarah Cannon Research Institute in Nashville, Tennessee with a second center in Sarasota, Florida. It is our intention to open one or more additional clinical sites in the near future.
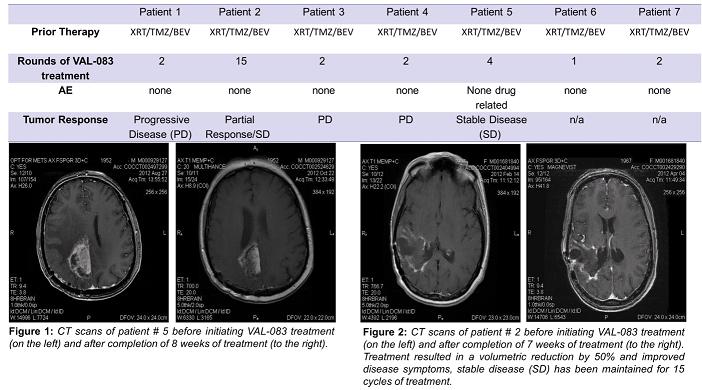
We presented initial data from our clinical trial in November 2012 at the Society for NeuroOncology annual meeting. A summary of these data is shown in the table below. The data support that VAL-083 is safe and well tolerated with no drug-related adverse events (AE) observed to date. An overall response rate of 28.5%, where tumor growth had stabilized or regressed, has been observed at doses investigated to date. In the past, the FDA has approved drugs to treat GBM with a response rate of less than 20%.
While these data with VAL-083 are interim in nature, we believe the results to date demonstrate a strong potential for successful development of VAL-083 as a chemotherapy for the treatment of GBM. We plan to continuing working with our clinical investigators to determining an optimal dosing regimen for future registration trials.
VAL-083 in Leukemia and Hematologic Cancers
CML, also known as chronic myeloid leukemia, is a cancer of the white blood cells. The incidence of CML in the United States is approximately two per 100,000 population.
CML is characterized by three progressive phases: chronic, aggressive and blast, each corresponding with poorer prognosis. Approximately 85% of patients with CML are in the chronic phase at the time of diagnosis. Chronic phase patients are usually asymptomatic or have only mild symptoms such as fatigue or no symptoms at all. The duration of chronic phase is variable and depends on how early the disease was diagnosed as well as type of treatment. Without treatment, CML progresses to an accelerated phase and eventually to blast crisis. Blast crisis is the final phase in the evolution of CML and behaves like an acute leukemia with rapid progression and short expected survival.
VAL-083 has shown promise in CML in multiple pre-clinical and clinical studies. The NCI studied VAL-083 extensively in laboratory and animal models of hematological malignancies (blood cancers). VAL-083 is approved by the SFDA for the treatment of CML in China. While VAL-083 maintains labeling for CML in China, use of the drug in the modern era has been limited by a preference for targeted therapies such as tyrosine kinase inhibitors (TKIs).
TKIs have become the standard of care for CML and non-small cell lung cancer (NSCLC). TKI therapy has resulted in vastly improved outcomes; however, patients often develop resistance to TKI therapy. Recent evidence proposes unique mechanisms of resistance in patients of East Asian descent who experience significantly inferior responses to TKIs, including imatinib (Gleevec®) in CML and erlotinib (Tarceva®) in lung cancer.
We believe that data from NCI-sponsored studies and commercial evidence from the Chinese market support substantive clinical benefit of VAL-083 in CML. We also believe that the unique mechanism of action of VAL-083, in combination with newly developed data positions the drug as a valuable therapy for patients who have failed other treatments, including TKIs. This represents a significant clinical and commercial opportunity for large subsets of patient populations in the existing-approved China market as well as for global development in CML.
Based on these beliefs, we have acquired commercial rights to VAL-083 in China where it is approved for the treatment of CML and Lung Cancer. We have also developed new non-clinical data demonstrating that VAL-083 is active against TKI-resistant CML. We have begun to establish a network of leading oncologists to develop new clinical and non-clinical data which will demonstrate the clinical utility of VAL-083 in CML patients who are resistant to TKIs. We believe this strategy will result in sales growth for VAL-083 in China and generate near-term revenue for our company through sales and marketing partnerships as well as position VAL-083 for global development in CML.
In addition, we plan to investigate VAL-083 as a potential treatment for other types of blood cancer. Acute Myeloid Leukemia (AML) and Acute Lymphoblastic Leukemia (ALL) are of particular interest based on published data and lack of effective therapeutic options. We have initiated preliminary discussions with leading cancer centers regarding the development of a clinical strategy for the development of VAL-083 in other types of blood cancer.
VAL-083 in Lung Cancer
Lung cancer is characterized as small cell and non-small cell lung cancer (NSLSC). NSCLC is the most common type of lung cancer.
There are three common forms of NSCLC: adenocarcinomas are often found in an outer area of the lung; squamous cell carcinomas are usually found in the center of the lung next to an air tube (bronchus); and large cell carcinomas, which can occur in any part of the lung and tend to grow and spread faster than adenocarcinoma.
Smoking is the most important risk factor in the development of lung cancer. According to the World Cancer Report (2008), 21% of cancer deaths are related to smoking, especially lung cancer. Additionally, high levels of air pollution have been implicated as significant causes of lung cancer. Incidence of lung cancer in the United States is approximately 59 per 100,000 with the majority (52:100,000) being NSLSC.
According to The Nationwide Nutrition and Health Survey (2002), China has the world’s largest smoking population, with a smoking rate of 24.0% on average (50.2% for men and 2.8% for women), and a total number of 350 million smokers. The World Health Organization reports that the incidence of lung cancer in China is 34 per 100,000 population; however, some estimates are much higher exceeding 120 per 100,000 population for males aged 55-60 in urban areas.
According to an exhaustive survey conducted by the Chinese Ministry of Health and the Ministry of Science and Technology, smoking, poor diet, water pollution and environmental problems have caused the nation's cancer death rate to rise 80 percent in the past 30 years and cancer is now accountable for 25 percent of all urban deaths and 21 percent of all rural deaths. Based on these trends, the World Health Organization projects that the incidence of lung cancer in China is expected to exceed one million (1,000,000) new cases per year by 2025.
Similar to CML treatment, TKIs are standard front-line therapy in NSCLC; however resistance to TKI therapy is common in lung cancer patients. It has also been reported that cigarette smoke may directly induce resistance to TKIs. This factor could further exacerbate resistance to modern targeted therapies in populations such as China where smoking is highly prevalent. In addition, the same East-Asian specific resistance linked to TKI-resistance in CML has been shown to correlate with TKI-resistance in NSLSC.
The activity of VAL-083 against lung cancer was studied extensively by the NCI. VAL-083 demonstrated activity against NSCLC in laboratory and animal studies. VAL-083 was also investigated in a number of clinical trials in the United States and Europe during the 1970s both as a stand-alone therapy and in combination with other chemotherapeutic regimens. VAL-083 is approved by the SFDA for the treatment of lung cancer in China; however, DelMar believes that the use of the drug in the modern era has been limited by a preference for targeted therapies such as TKIs.
We believe VAL-083’s unique bi-functional alkylating mechanism of action could make it a valuable drug of choice in NSCLC patients who are or become resistant to TKI therapy. In addition, VAL-083 readily crosses the blood brain barrier suggesting that it may be possible for VAL-083 to treat patients whose lung cancer has spread to the brain.
Based on these beliefs, we have acquired commercial rights to VAL-083 in China where it is approved for the treatment of lung cancer. We plan to work with leading oncologists to develop new clinical and non-clinical data which will demonstrate the clinical utility of VAL-083 in NSCLC patients who are resistant to TKIs. We believe this strategy will result in sales growth for VAL-083 in China and generate near-term revenue for our company through sales and marketing partnerships as well as position VAL-083 for global development in lung cancer.
VAL-083 Target Markets
We are targeting cancer indications which we believe represent market opportunities in the hundreds of millions of dollars in North America and potentially in the billions of dollars worldwide. The pharmaceutical industry, in general, is a highly profitable, highly innovative industry. In 2006, the global pharmaceutical industry generated over $640 billion dollars in revenue. According to published reports, global pharmaceutical sales are highly stratified by region, with North America, the European Union and Japan accounting for 55% of global pharmaceutical sales in 2009; however, the most rapid growth in the sector is from developing countries, particularly China.
|
Glioblastoma Multiforme (GBM): Newly diagnosed patients suffering from GBM are initially treated through invasive brain surgery, although disease progression following surgical resection is nearly 100%. Temozolomide (Temodar®) in combination with radiation is the front-line therapy for GBM following surgery. Temodar currently generates more than US$950 million annually in global revenues even though most patients fail to gain long-term therapeutic benefits. Approximately 60% of GBM patients treated with Temodar experience tumor progression within one year.
Bevacizumab (Avastin®) has been approved for the treatment of GBM in patients failing Temodar®. In clinical studies, only about 20% of patients failing Temodar respond to Avastin therapy. In spite of these low efficacy results, treatment of GBM in North America alone is projected to add US$200 million annually to the revenues of Avastin with projected growth in GBM to US$650 million by 2016.
Approximately 48% of patients who are diagnosed with GBM will fail both front-line therapy and Avastin. Based on disease incidence, we believe the market for treating GBM patients the post-Avastin failure exceeds US$200 million annually in North America. Subject to successfully completing clinical trials and obtaining approval by the FDA and other applicable regulatory agencies globally, we also believe that VAL-083 could potentially generate sales in excess of $1 billion world-wide as a potential front-line therapy for GBM.
Leukemia: The potential of VAL-083 in the treatment of CML has been established in both human clinical trials conducted by the NCI and by the drug’s commercial approval in China. The Tyrosine Kinase Inhibitor Gleevec® is currently used as front-line therapy in the treatment of CML currently achieves global revenue in excess of $1 billion annually. We believe that VAL-083 has potential to capture a portion of the CML market through demonstration of activity in TKI-resistant CML patients. We also believe that VAL-083 may offer significant commercial opportunities through the treatment of other types of blood cancer such as AML or ALL.
Lung Cancer: The potential of VAL-083 in the treatment of NSLSC has been established in both human clinical trials conducted by the NCI and by the drug’s commercial approval in China. A 2012 report published by Decision Resources, Inc. (http://decisionresources.com/), forecasts that the NSCLC drug market will exceed US$4 billion in 2015.
|
 |
VAL-083 Manufacturing
VAL-083 is currently manufactured in accordance with State Food and Drug Administration (SFDA) and Chinese Pharmacopoeia guidelines to ensure drug quality control, drug use safety, and drug efficacy. Approval by the U.S. Food and Drug Administration (“FDA”) will require VAL-083 and other products developed by us to be manufactured in accordance with United States Pharmacopeia (USP) in accordance with Current Good Manufacturing Practice (cGMP) regulations. cGMP provides for systems that assure proper design, monitoring, and control of manufacturing processes and facilities. Adherence to the cGMP regulations assures the identity, strength, quality, and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations.
We have established an exclusive purchasing relationship with the Chinese manufacturer that has enabled us to obtain drug product for human clinical trials in the United States and commercial rights in China. The Chinese manufacturer has established a commercial-scale manufacturing process based on the North American process originally developed for the NCI.
Ensuring a viable long-term supply of the VAL-083 drug product suitable for registration and commercialization in North America and Europe will require investment in improved manufacturing and quality controls. We will seek to build upon our expertise and our intellectual property related to the existing manufacturing processes for VAL-083 in collaboration with the current manufacturer to allow compliance with cGMP. In addition, we have identified third party contract manufacturers with the capabilities to establish the processes, procedures and quality systems necessary to meet U.S., Canadian, E.U. and other international cGMP manufacturing requirements. Such requirements include strong quality management systems, obtaining appropriate quality raw materials, establishing robust operating procedures, detecting and investigating product quality deviations, and maintaining reliable testing laboratories.
Patents & Proprietary Rights
Our success will depend in part on our ability to protect our existing product candidates and the products we acquire or license by obtaining and maintaining a strong proprietary position. To develop and maintain our position, we intend to continue relying upon patent protection, orphan drug status, Hatch-Waxman exclusivity, trade secrets, know-how, continuing technological innovations and licensing opportunities. We intend to seek patent protection whenever available for any products or product candidates and related technology we acquire in the future.
The molecule described as VAL-083 is not currently covered by any issued patents. We have filed new patent applications covering VAL-083 where we have claimed the use of and improvements related VAL-083 and other novel aspects of our proposed treatment regimen. We have also developed and filed patents on manufacturing process improvements for VAL-083. In addition, we plan to implement strategies which may enable us to acquire patent protection for the formulation and composition of the active pharmaceutical ingredient and finished dosage form of VAL-083 products.
We may also seek orphan drug status whenever it is available. If a product which has an orphan drug designation subsequently receives the first regulatory approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, meaning that the applicable regulatory authority may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for a period of seven years in the U.S. and Canada, and 10 years in the E.U. Orphan drug designation does not prevent competitors from developing or marketing different drugs for the same indication or the same drug for a different clinical indication. In February 2012, we announced that the FDA has granted orphan drug status to VAL-083.
Under the Hatch-Waxman Amendments, newly approved drugs and indications benefit from a statutory period of non-patent marketing exclusivity. These amendments provide five-year data exclusivity to the first applicant to gain approval of an NDA for a new chemical entity, meaning that the FDA has not previously approved any other new drug containing the same active ingredient. The Hatch-Waxman Amendments prohibit the submission of an abbreviated new drug application, also known as an ANDA or generic drug application, during the five-year exclusive period if no patent is listed. If there is a patent listed and the ANDA applicant certifies that the NDA holder’s listed patent for the product is invalid or will not be infringed, the ANDA can be submitted four years after NDA approval. Protection under the Hatch-Waxman Amendments will not prevent the filing or approval of another full NDA; however, the applicant would be required to conduct its own pre-clinical studies and adequate and well-controlled clinical trials to demonstrate safety and effectiveness. The Hatch-Waxman Amendments also provide three years of data exclusivity for the approval of NDAs with new clinical trials for previously approved drugs and supplemental NDAs, for example, for new indications, dosages or strengths of an existing drug, if new clinical investigations were conducted by or on behalf of the sponsor and were essential to the approval of the application. This three-year exclusivity covers only the new changes associated with the supplemental NDA and does not prohibit the FDA from approving ANDAs for drugs containing the original active ingredient. We intend to rely on the Hatch-Waxman Amendments for five years of data exclusivity for VAL-083.
We also rely on trade secret protection for our confidential and proprietary information. We believe that the substantial costs and resources required to develop technological innovations, such as the manufacturing processes associated with VAL-083, will help us to protect the competitive advantage of our product candidates.
It is our policy to require our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual shall be our exclusive property.
Government Regulation and Product Approval
Regulation by governmental authorities in the U.S. and other countries is a significant factor, affecting the cost and time of our research and product development activities, and will be a significant factor in the manufacture and marketing of any approved products. All of our products require regulatory approval by governmental agencies prior to commercialization. In particular, our products are subject to rigorous pre-clinical and clinical testing and other approval requirements by the FDA and similar regulatory authorities in other countries. Various statutes and regulations also govern or influence the manufacturing, safety, reporting, labeling, transport and storage, record keeping and marketing of our products. The lengthy process of seeking these approvals, and the subsequent compliance with applicable statutes and regulations, require the expenditure of substantial resources. Any failure by us to obtain, or any delay in obtaining, the necessary regulatory approvals could harm our business.
The regulatory requirements relating to the testing, manufacturing and marketing of our products may change from time to time and this may impact our ability to conduct clinical trials and the ability of independent investigators to conduct their own research with support from us.
The clinical development, manufacturing and marketing of our products are subject to regulation by various authorities in the U.S., the E.U. and other countries, including, in the U.S., the FDA, in Canada, Health Canada, and, in the E.U., the EMEA. The Federal Food, Drug, and Cosmetic Act, the Public Health Service Act in the U.S. and numerous directives, regulations, local laws and guidelines in Canada and the E.U. govern the testing, manufacture, safety, efficacy, labeling, storage, record keeping, approval, advertising and promotion of our products. Product development and approval within these regulatory frameworks takes a number of years and involves the expenditure of substantial resources.
Regulatory approval will be required in all the major markets in which we seek to develop our products. At a minimum, approval requires the generation and evaluation of data relating to the quality, safety, and efficacy of an investigational product for its proposed use. The specific types of data required and the regulations relating to this data will differ depending on the territory, the drug involved, the proposed indication and the stage of development.
In general, new chemical entities are tested in animals until adequate evidence of safety is established to support the proposed clinical study protocol designs. Clinical trials for new products are typically conducted in three sequential phases that may overlap. In Phase I, the initial introduction of the pharmaceutical into either healthy human volunteers or patients with the disease (20 to 50 subjects), the emphasis is on testing for safety (adverse effects), dosage tolerance, metabolism, distribution, excretion and clinical pharmacology. Phase II involves studies in a limited patient population (50 to 200 patients) to determine the initial efficacy of the pharmaceutical for specific targeted indications, to determine dosage tolerance and optimal dosage and to identify possible adverse side effects and safety risks. Once a compound shows preliminary evidence of some effectiveness and is found to have an acceptable safety profile in Phase II evaluations, Phase III trials are undertaken to more fully evaluate clinical outcomes in a larger patient population in adequate and well-controlled studies designed to yield statistically sufficient clinical data to demonstrate efficacy and safety.
In the U.S., specific pre-clinical data, manufacturing and chemical data, as described above, need to be submitted to the FDA as part of an IND application, which, unless the FDA objects, will become effective 30 days following receipt by the FDA. Phase I studies in human volunteers may commence only after the application becomes effective. Prior regulatory approval for human healthy volunteer studies is also required in member states of the E.U. Currently, in each member state of the E.U., following successful completion of Phase I studies, data are submitted in summarized format to the applicable regulatory authority in the member state in respect of applications for the conduct of later Phase II studies. The regulatory authorities in the E.U. typically have between one and three months in which to raise any objections to the proposed study, and they often have the right to extend this review period at their discretion. In the U.S., following completion of Phase I studies, further submissions to regulatory authorities are necessary in relation to Phase II and III studies to update the existing IND. Authorities may require additional data before allowing the studies to commence and could demand that the studies be discontinued at any time if there are significant safety issues. In addition to the regulatory review, a study involving human subjects has to be approved by an independent body. The exact composition and responsibilities of this body will differ from country to country. In the U.S., for example, each study will be conducted under the auspices of an independent institutional review board at each institution at which the study is conducted. This board considers among other things, the design of the study, ethical factors, the privacy of protected health information as defined under the Health Insurance Portability and Accountability Act, the safety of the human subjects and the possible liability risk for the institution. Equivalent rules to protect subjects’ rights and welfare apply in each member state of the E.U. where one or more independent ethics committees, which typically operate similarly to an institutional review board, will review the ethics of conducting the proposed research. Other regulatory authorities around the rest of the world have slightly differing requirements involving both the execution of clinical trials and the import/export of pharmaceutical products. It is our responsibility to ensure we conduct our business in accordance with the regulations of each relevant territory.
By leveraging existing pre-clinical and clinical data, we are seeking build upon an existing pre-clinical and clinical safety and efficacy database to accelerate our research. In addition, our focus on end-stage population which has no current treatment options, commercialization may be achieved in an accelerated manner. Approval by the FDA in this category generally has been based on objective response rates and duration of responses rather than demonstration of survival benefit. As a result, trials of drugs to treat end-stage refractory cancer indications have historically involved fewer patients and generally have been faster to complete than trials of drugs for other indications. We are aware that the FDA and other similar agencies are regularly reviewing the use of objective endpoints for commercial approval and that policy changes may impact the size of trials required for approval, timelines and expenditures significantly.
In order to gain marketing approval we must submit a dossier to the relevant authority for review, which is known in the U.S. as an NDA and in the E.U. as a marketing authorization application, or MAA. The format is usually specific and laid out by each authority, although in general it will include information on the quality of the chemistry, manufacturing and pharmaceutical aspects of the product as well as the non-clinical and clinical data. Once the submitted NDA is accepted for filing by the FDA, it undertakes the review process that takes 10 months, unless an expedited priority review is granted which takes six months to complete. Approval can take several months to several years, if multiple 10-month review cycles are needed before final approval is obtained, if at all.
The approval process can be affected by a number of factors. The NDA may be approvable requiring additional pre-clinical, manufacturing data or clinical trials which may be requested at the end of the 10 month NDA review cycle, thereby delaying marketing approval until the additional data are submitted and may involve substantial unbudgeted costs. The regulatory authorities usually will conduct an inspection of relevant manufacturing facilities, and review manufacturing procedures, operating systems and personnel qualifications. In addition to obtaining approval for each product, in many cases each drug manufacturing facility must be approved. Further inspections may occur over the life of the product. An inspection of the clinical investigation sites by a competent authority may be required as part of the regulatory approval procedure. As a condition of marketing approval, the regulatory agency may require post-marketing surveillance to monitor for adverse effects or other additional studies as deemed appropriate. After approval for the initial indication, further clinical studies are usually necessary to gain approval for any additional indications. The terms of any approval, including labeling content, may be more restrictive than expected and could affect the marketability of a product.
The FDA offers a number of regulatory mechanisms that provide expedited or accelerated approval procedures for selected drugs in the indications on which we are focusing our efforts. These include accelerated approval under Subpart H of the agency’s NDA approval regulations, fast track drug development procedures and priority review. At this time, we have not determined whether any of these approval procedures will apply to any of our current drug candidates.
The U.S., E.U. and other jurisdictions may grant orphan drug designation to drugs intended to treat a “rare disease or condition,” which, in the U.S., is generally a disease or condition that affects no more than 200,000 individuals. In the E.U., orphan drug designation can be granted if: the disease is life threatening or chronically debilitating and affects no more than 50 in 100,000 persons in the E.U.; without incentive it is unlikely that the drug would generate sufficient return to justify the necessary investment; and no satisfactory method of treatment for the condition exists or, if it does, the new drug will provide a significant benefit to those affected by the condition. If a product that has an orphan drug designation subsequently receives the first regulatory approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, meaning that the applicable regulatory authority may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for a period of seven years in the U.S. and 10 years in the E.U. Orphan drug designation does not prevent competitors from developing or marketing different drugs for the same indication or the same drug for different indications. Orphan drug designation must be requested before submitting an NDA or MAA. After orphan drug designation is granted, the identity of the therapeutic agent and its potential orphan use are publicly disclosed. Orphan drug designation does not convey an advantage in, or shorten the duration of, the review and approval process; however, this designation provides an exemption from marketing authorization (NDA) fees.
We are also subject to numerous environmental and safety laws and regulations, including those governing the use and disposal of hazardous materials. The cost of compliance with and any violation of these regulations could have a material adverse effect on our business and results of operations. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards prescribed by state and federal regulations, accidental contamination or injury from these materials may occur. Compliance with laws and regulations relating to the protection of the environment has not had a material effect on our capital expenditures or our competitive position. However, we are not able to predict the extent of government regulation, and the cost and effect thereof on our competitive position, which might result from any legislative or administrative action pertaining to environmental or safety matters.
Competition
The development and commercialization of new drugs is highly competitive and we may face competition established pharmaceutical and biotechnology companies, as well as from academic institutions, government agencies and private and public research institutions worldwide.
Various products currently are marketed for the treatment of GBM and other cancers that we may target with our product candidates and a number of companies are developing new treatments. Companies also developing products for GBM include but are not limited to Celgene Corp., Cell Therapeutics, Inc., Exelixis, Inc., YM Biosciences Inc, and many major pharmaceutical companies. Our success will be based in part on our ability to build and actively manage a portfolio of drugs that addresses unmet medical needs and create value in patient therapy.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Our commercial opportunity will be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects or are less expensive than products that we may develop. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business.
We expect that our ability to compete effectively will depend upon our ability to:
|
●
|
successfully and rapidly complete adequate and well-controlled clinical trials that demonstrate statistically significant safety and efficacy and to obtain all requisite regulatory approvals in a cost-effective manner;
|
|
●
|
maintain a proprietary position for our manufacturing processes and other technology;
|
|
●
|
attract and retain key personnel; and
|
|
●
|
build an adequate sales and marketing infrastructure for any approved products.
|
Failure to do one or more of these activities could have an adverse effect on our business, financial condition or results of operations.
Employees
We currently do not have full-time employees, but retain the services of approximately 19 persons on an independent contractor/consultant and contract-employment basis. As such, currently operate in a “virtual” corporate structure in order to minimize fixed personnel costs. Over time, we plan to establish a base of full time employees and corporate infrastructure. We anticipate that in the near future, key personnel, including Jeffrey Bacha, our chief executive officer, and Dr. Dennis Brown, our chief scientific officer, will enter into employment agreements with the Company on customary terms.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
This Management Discussion and Analysis (MD&A) contains “forward-looking statements”, which represent our projections, estimates, expectations or beliefs concerning among other things, financial items that relate to management’s future plans or objectives or to our future economic and financial performance. In some cases, you can identify these statements by terminology such as “may”, “should”, “plans”, “believe”, “will”, “anticipate”, “estimate”, “expect” “project”, or “intend”, including their opposites or similar phrases or expressions. You should be aware that these statements are projections or estimates as to future events and are subject to a number of factors that may tend to influence the accuracy of the statements. These forward-looking statements should not be regarded as a representation by the Company or any other person that the events or plans of the Company will be achieved. You should not unduly rely on these forward-looking statements, which speak only as of the date of this MD&A. Except as may be required under applicable securities laws, we undertake no obligation to publicly revise any forward-looking statement to reflect circumstances or events after the date of this MD&A or to reflect the occurrence of unanticipated events. You should, however, review the factors and risks we describe under “Risk Factors” in this report. Actual results may differ materially from any forward looking statement.
Overview
DelMar (BC) is a British Columbia, Canada corporation founded in 2010. We are a clinical and commercial stage drug development company with a focus on the treatment of cancer. Our mission is to benefit patients and create shareholder value by rapidly developing and commercializing anti-cancer therapies in orphan cancer indications where patients have failed modern therapy.
Our drug discovery research focuses on identifying well-validated clinical and commercial-stage compounds and establishing a scientific rationale for development in modern orphan drug indications. Promising candidates are further researched through our network of consultants and contract research organizations. This approach allows us to rapidly identify and advance potential drug candidates without significant investment in “wet lab” infrastructure. Based on this strategy, we acquired intellectual property and prototype drug product related to our lead drug candidate, VAL-083, from Valent in September 2010 and have identified multiple additional drug candidates that we may have the opportunity to license or acquire in the future.
VAL-083
Central Nervous System Cancers
Our lead product candidate, VAL-083, represents a “first in class” small-molecule chemotherapeutic. The molecular structure of VAL-083 is not an analogue or derivative of other small molecule chemotherapeutics approved for the treatment of cancer. VAL-083 has been assessed in multiple clinical studies sponsored by the National Cancer Institute (“NCI”) in the United States as a treatment against various cancers including lung, brain, cervical, ovarian tumors and leukemia. Published pre-clinical and clinical data suggest that VAL-083 may be active against a range of tumor types. VAL-083 is approved as a cancer chemotherapeutic in China for the treatment of chronic myelogenous leukemia and lung cancer. VAL-083 has not been approved for any indications outside of China.
Upon obtaining regulatory approval, we intend to commercialize VAL-083 and other product candidates for the treatment of orphan and cancer indications where patients have failed other therapies or have limited medical options. Orphan diseases are defined in the United States under the Rare Disease Act of 2002 as “any disease or condition that affects less than 200,000 persons in the United States”. The Orphan Drug Act of 1983 is a federal law that provides financial and other incentives including a period of market exclusivity to encourage the development of new treatments for orphan diseases.
We research the mechanism of action of our product candidates to determine the clinical indications best suited for therapy and rapidly advance our product candidates into human clinical trials and toward commercialization. In October 2011, we initiated clinical trials with VAL-083 as a potential new treatment for GBM, the most common and aggressive form of brain cancer. In April 2012, we presented data at the American Association of Cancer Research annual meeting demonstrating that VAL-083 maintains activity in tumors resistant to the current front-line GBM therapy, Temodar®. In November 2012, we presented interim data from our clinical trial at the Annual Meeting of the Society for NeuroOncology demonstrating that VAL-083 can shrink or halt the growth of tumors in brain cancer patients who have failed other approved treatments. Currently, there is no approved therapy for these patients.
In addition to our clinical development activities in the United States, we have obtained exclusive commercial rights to VAL-083 in China. In October 2012, we announced that we had entered into a collaboration agreement with the only manufacturer licensed by the SFDA to produce the product for the China market. This agreement provides us with exclusive commercial rights which position us to generate near-term revenue through product sales or royalties for its approved indications in China while we seek global approval in new indications.
VAL-083 was originally discovered in the 1960’s. We have filed a broad portfolio of new patent applications to protect our intellectual property. Our patent applications claim compositions and methods related to the use of VAL-083 and related compounds as well as methods of synthesis and quality controls for the manufacturing process of VAL-083. We announced that VAL-083 has been granted Orphan Drug protection for the treatment of glioma, including GBM by the FDA in the United States and the EMA in February 2012 and January 2013, respectively. Orphan drugs generally follow the same regulatory development path as any other pharmaceutical product. However, incentives such as scientific advice and reduction or waiver of registration fees and access to specialized grant funding may be available to support and accelerate development of orphan drug candidates. In addition, DelMar (BC) may sell VAL-083 as a treatment for glioma without competition for seven years in the United States and for ten years in the European Union following market approval, in respect of a medicinal product containing a similar active substance for the same indication.
The activity of VAL-083 against solid tumors, including lung cancer, has been established in both pre-clinical and human clinical trials conducted by the NCI and by the drug's commercial approval in China. Decision Resources, Inc., forecasts that the NSCLC drug market will exceed USD $4.1 billion in 2012. We plan to establish a strong scientific and clinical rationale to support out-licensing activities to unlock the potential value of the drug in partnership with larger pharmaceutical companies with the resources and commercial infrastructure to effectively develop and launch a lung cancer product.
Additional Orphan Drug Indications
We have established a high-level scientific rationale for the development of VAL-083 in additional high-value orphan cancer indications. Acute Myeloid Leukemia (“AML”) is of particular interest based on published human clinical data and lack of effective therapeutic options. We have initiated preliminary discussions with leading cancer researchers regarding the development of a clinical strategy for the development of VAL-083 in AML.
Developing Partnerships with Pharmaceutical Companies
Guangxi Wuzhou Pharmaceutical Company
DelMar (BC) has a strategic collaboration with Guangxi Wuzhou Pharmaceutical Company, a subsidiary of publicly traded Guangxi Wuzhou Zhongheng Group Co., Ltd. for the development of VAL-083 (marketed as “DAG” in China). VAL-083 is approved by the SFDA as a cancer chemotherapy for the treatment of Chronic Myelogenous Leukemia (“CML”) and lung cancer. Guangxi Wuzhou Pharmaceuticals is licensed by the SFDA to manufacture and sell VAL-083 in China for these indications.
DelMar (BC) and Guangxi Wuzhou Pharmaceuticals plan to use new data being generated through DelMar’s clinical programs to expand the market in China and to seek regulatory approval for the drug in multiple indications on a global basis. The collaboration expands the exclusive supply relationship between DelMar and Guangxi Wuzhou Pharmaceuticals to include the Chinese market and all markets outside China. The companies will work together to insure the product specifications meet global standards in order to accelerate international development and regulatory approval. Guangxi Wuzhou Pharmaceuticals will provide funding for clinical trials conducted in China and will be the exclusive supplier of DAG for injection and DelMar will be responsible for development and commercialization.
Reverse Acquisition
On January 25, 2013, we entered into and closed the Exchange Agreement (see Item 1.01).
As a result of the shareholders of DelMar (BC) having a controlling interest in DelMar Pharmaceuticals, Inc. subsequent to the transaction, for accounting purposes the transaction constitutes a reverse recapitalization with DelMar (BC) being the accounting acquirer even though legally DelMar (BC) is the acquiree. Therefore, the net assets of DelMar Pharmaceuticals, Inc. are recorded at fair value at the date of the transaction. No goodwill is recorded with respect to the transaction as it does not constitute a business combination.
Unit Offering
On January 25, 2012, the Company entered into and closed the Private Offering (see Item 1.01).
Related Parties
We acquired our VAL-083 prototype drug, patents and technology rights from Valent. In addition, Valent has incurred a significant portion of our clinical expenses during the period ended December 31, 2011 and has in turn invoiced us for those expenses. One of our officers and directors is also a Principal of Valent and as result Valent is a related party to us.
Valent Royalty Reduction Agreement
On January 21, 2013, Valent agreed to reduce its royalties on future sales of VAL-083 in exchange for 1,150,000 common shares of the Company.
Derivative Liability
Based on the terms of the warrants issued as part of our units issued during the year ended December 31, 2011, we determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value every reporting period with gains or losses on the changes in fair value recorded in the statement of loss and comprehensive loss.
For the Nine Months Ended September 30, 2012 Compared to the Nine Months Ended September 30, 2011
Selected Financial Information
The financial information reported here in has been prepared in accordance with US GAAP. Our functional currency is the Canadian dollar (“CDN”) but we report our results in United States Dollars (“USD”). The following table represents selected financial information for our three and nine months ended September 30, 2012 and 2011.
Selected Balance Sheet Data
| |
|
September 30,
2012
$
|
|
|
December 31,
2011
$
|
|
| |
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
|
55,300 |
|
|
|
15,018 |
|
|
Working capital (deficiency)
|
|
|
(604,955 |
) |
|
|
(770,987 |
) |
|
Total Assets
|
|
|
159,291 |
|
|
|
68,017 |
|
|
Derivative liability
|
|
|
(354,662 |
) |
|
|
(106,146 |
) |
|
Total shareholders’ deficiency
|
|
|
(959,617 |
) |
|
|
(877,133 |
) |
Liquidity and Capital Resources
| |
|
September 30,
2012
$
|
|
|
December 31,
2011
$
|
|
|
Change
$
|
|
|
Change
%
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
|
55,300 |
|
|
|
15,018 |
|
|
|
40,282 |
|
|
|
268 |
|
|
Current assets
|
|
|
159,291 |
|
|
|
68,017 |
|
|
|
91,274 |
|
|
|
134 |
|
|
Current liabilities
|
|
|
(764,246 |
) |
|
|
(839,004 |
) |
|
|
74,758 |
|
|
|
(9 |
) |
|
Working capital (deficiency)
|
|
|
(604,955 |
) |
|
|
(770,987 |
) |
|
|
166,032 |
|
|
|
22 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended September 30,
2012
$
|
|
|
Nine Months Ended September 30,
2011
$
|
|
|
Change
$
|
|
|
Change
%
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash used in operating activities
|
|
|
(631,288 |
) |
|
|
(65,415 |
) |
|
|
(565,873 |
) |
|
|
865 |
|
|
Cash flows from financing activities
|
|
|
671,570 |
|
|
|
100,056 |
|
|
|
571,514 |
|
|
|
571 |
|
Comparison of Cash Flow
Operating Activities
Net cash used in operating activities increased to $631,288 for the nine months ended September 30, 2012 from $65,415 for the nine months ended September 30, 2011. The increase was partially the result of an increase in the net loss to $1,977,084 for the nine months ended September 30, 2012 compared to $796,947 for the nine months ended September 30, 2011. The most significant change in non-cash working capital between the two periods was a change in accounts payable and accrued liabilities. For the nine months ended September 30, 2012 there was an inflow of $97,739 from an increase in accounts payable and accrued liabilities compared to an inflow of $478,643 for the nine months ended September 30, 2011. Partially offsetting the impact of the higher net loss and changes in working capital were non-cash items totaling $1,218,996 incurred in the current period consisting of interest, units issued for services, shares issued for services, warrants issued for services, and share-based compensation. The only non-cash item from the prior period was interest $5,053 and $190,690 for share-based payments.
Financing Activities
We received $671,570 in net proceeds from the issuance of units during the nine months ended September 30, 2012 compared to $100,056 in proceeds from the issuance of shares during the nine months ended September 30, 2011.
Operating Capital and Capital Expenditure Requirements
For the nine months ended September 30, 2012, we reported a loss of $1,977,084 and an accumulated deficit of $3,418,854 at that date. As at September 30, 2012, DelMar (BC) had cash and cash equivalents on hand of $55,300 and a negative working capital balance of $604,955. DelMar (BC) does not have the prospect of achieving revenues in the near future and DelMar (BC) will require additional funding to maintain its research and development projects and for general operations. These circumstances lend substantial doubt as to the ability of DelMar (BC) to meet its obligations as they come due.
Consequently, management is pursuing various financing alternatives to fund our operations so we can continue as a going concern. In addition, we have not begun to commercialize or generate revenues from any product candidate. Accordingly, we are considered to be in the development stage as defined in Accounting Standards Codification (ASC) 915-10. Management plans to secure the necessary financing through the issue of new equity and/or the entering into of strategic partnership arrangements. Nevertheless, there is no assurance that these initiatives will be successful.
The September 30, 2012 financial statements have been prepared on a going concern basis which assumes that DelMar (BC) will continue its operations for the foreseeable future and contemplates the realization of assets and the settlement of liabilities in the normal course of business.
The conditions and risks noted above cast substantial doubt on the validity of that assumption. The September 30, 2012 financial statements do not give effect to any adjustments to the amounts and classification of assets and liabilities that may be necessary and could potentially be material, should DelMar (BC) be unable to continue as a going concern.
Our future funding requirements will depend on many factors, including but not limited to:
|
|
● the rate of progress and cost of our clinical trials, preclinical studies and other discovery and research and development activities;
|
|
|
● the costs associated with establishing manufacturing and commercialization capabilities;
|
|
|
● the costs of acquiring or investing in businesses, product candidates and technologies;
|
|
|
● the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
|
|
|
● the costs and timing of seeking and obtaining FDA and other regulatory approvals;
|
|
|
● the effect of competing technological and market developments; and
|
|
|
● the economic and other terms and timing of any collaboration, licensing or other arrangements into which we may enter.
|
Until we can generate a sufficient amount of product revenue to finance our cash requirements, which we may never do, we expect to finance future cash needs primarily through public or private equity offerings, debt financings or strategic collaborations. Although we are not reliant on institutional credit finance and therefore not subject to debt covenant compliance requirements or potential withdrawal of credit by banks, the current economic climate has also impacted the availability of funds and activity in equity markets. We do not know whether additional funding will be available on acceptable terms, or at all. If we are not able to secure additional funding when needed, we may have to delay, reduce the scope of or eliminate one or more of our clinical trials or research and development programs or make changes to our operating plan. In addition, we may have to partner one or more of our product candidate programs at an earlier stage of development, which would lower the economic value of those programs to us.
Selected Statement of Operations Data
| |
|
For the Three Months Ended |
For the Nine Months Ended |
|
| |
|
September 30,
2012
$
|
|
|
September 30,
2011
$
|
|
|
September 30,
2012
$
|
|
|
September 30,
2011
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
229,488 |
|
|
|
272,462 |
|
|
|
1,217,021 |
|
|
|
620,469 |
|
|
General and administrative
|
|
|
218,732 |
|
|
|
61,279 |
|
|
|
781,324 |
|
|
|
127,833 |
|
|
Foreign exchange (gain) loss
|
|
|
(22,295 |
) |
|
|
41,738 |
|
|
|
(26,891 |
) |
|
|
32,965 |
|
|
Interest expense
|
|
|
1,900 |
|
|
|
10,628 |
|
|
|
5,630 |
|
|
|
15,680 |
|
|
Loss from operations
|
|
|
427,825 |
|
|
|
386,107 |
|
|
|
1,977,084 |
|
|
|
796,947 |
|
|
Weighted average number of shares outstanding
|
|
|
12,969,783 |
|
|
|
8,187,500 |
|
|
|
13,287,835 |
|
|
|
8,195,307 |
|
|
Loss per share
|
|
|
0.03 |
|
|
|
0.05 |
|
|
|
0.15 |
|
|
|
0.10 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comparison of the three months ended September 30, 2012 and 2011
| |
|
Three Months Ended
|
|
| |
|
September 30,
2012
$
|
|
|
September 30,
2011
$
|
|
|
Change
$
|
|
|
Change
%
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
229,488 |
|
|
|
272,462 |
|
|
|
(42,974 |
) |
|
|
(16 |
) |
|
General and administrative
|
|
|
218,732 |
|
|
|
61,279 |
|
|
|
157,453 |
|
|
|
257 |
|
|
Foreign exchange (gain) loss
|
|
|
(22,295 |
) |
|
|
41,738 |
|
|
|
(64,033 |
) |
|
|
(153 |
) |
|
Interest expense
|
|
|
1,900 |
|
|
|
10,628 |
|
|
|
(8,728 |
) |
|
|
(82 |
) |
|
Net loss
|
|
|
427,825 |
|
|
|
386,107 |
|
|
|
41,718 |
|
|
|
11 |
|
Research and Development
Research and development expenses decreased to $229,488 for the three months ended September 30, 2012 from $272,462 for the three months ended September 30, 2011. The largest components of research and development for both periods ended September 30 were clinical development expenses related to the clinical trials being undertaken with VAL-083. The clinical development costs were lower in the current period compared to the prior largely due to clinical preparation and start-up costs incurred in the three months ended September 30, 2011 compared to the three months ended September 30, 2012. Partially offsetting the impact of lower clinical development costs in 2012 compared to 2011 were higher contracted research and share-based payments incurred during the three months ended September 30, 2012 compared to the three months ended September 30, 2011. Contracted research costs were higher in the current period due to the initiation of pre-clinical research studies supporting new indications in the current period. There were no such preclinical studies on-going in the prior period. Share-based payments have increased partially due to stock option expenses as our first grant of stock options occurred in February 2012. Additionally, units were issued for services in both periods but for the three months ended September 30, 2012 agreements applicable to units for services covered the entire three months ended September 30, 2012 while in the three months ended September 30, 2011 units for services were applicable for only two months resulting in a lower expense in the prior period.
General and Administrative
General and administrative expenses were $218,732 for the three months ended September 30, 2012 compared to $61,279 for the three months ended September 30, 2011. The principal reasons for the increase were due to higher professional fees and share-based payments incurred in the current period compared to the prior period. The increase in professional fees related to costs incurred in the current period for the initiation of our first financial statement audit, legal fees related to the updating of our corporate records, and for business development fees incurred in relation to our collaboration in China and for activities relating to preparation for our financing expected to be completed in January 2013. Share-based payments have increased principally due to stock option expenses as our first grant of stock options occurred in February 2012. Additionally, units were issued for services in both periods but for the three months ended September 30, 2012 agreements applicable to units issued for services covered the entire three months ended September 30, 2012 while in the three months ended September 30, 2011 units for services were applicable for two months resulting in a lower expense in the prior period.
Foreign Exchange (Gain) Loss
Our functional currency is the CDN but we report our results in USD. The translation gains and losses are reported in other comprehensive loss/income. Foreign exchange gains and losses are the result of our incurring expenses in USD and then translating those USD expenses into CDN. We will continue to incur some expenses in USD and as a result will continue to be exposed to foreign exchange gains and losses in part because it is expected that the CDN will continue to be our functional currency.
We recognized a foreign exchange gain of $22,295 for the three months ended September 30, 2012 compared to a loss of $41,738 for the three months ended September 30, 2011. The change was due to changes in the exchange rate between the CDN and the USD and to varying levels of USD accounts payable.
Interest Expense
Pursuant to a loan agreement dated February 3, 2011, we obtained a loan from Valent in the amount of $250,000 for the purchase of the prototype drug product. The loan is unsecured and bears interest at 3.00% per year. As a result of the loan payable we recognized $1,900 respectively in accrued interest for each of the three month periods ended September 30, 2012 and 2011. During the three months ended September 30, 2011 we were was charged $8,728 in interest expense relating to outstanding trade payable balances.
Comparison of the nine months ended September 30, 2012 and 2011
| |
|
Nine Months Ended
|
|
|
|
|
|
|
|
| |
|
September 30,
2012
$
|
|
|
September 30,
2011
$
|
|
|
Change
$
|
|
|
Change
%
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
1,217,021 |
|
|
|
620,469 |
|
|
|
596,552 |
|
|
|
96 |
|
|
General and administrative
|
|
|
781,324 |
|
|
|
127,833 |
|
|
|
653,491 |
|
|
|
511 |
|
|
Foreign exchange (gain) loss
|
|
|
(26,891 |
) |
|
|
32,965 |
|
|
|
(59,856 |
) |
|
|
(182 |
) |
|
Interest expense
|
|
|
5,630 |
|
|
|
15,680 |
|
|
|
(10,050 |
) |
|
|
(64 |
) |
|
Net loss
|
|
|
1,977,084 |
|
|
|
796,947 |
|
|
|
1,180,137 |
|
|
|
148 |
|
Research and Development
Research and development expenses increased to $1,217,021 for the nine months ended September 30, 2012 from $620,469 for the nine months ended September 30, 2011. The largest component of research and development for the nine months ended September 30, 2012 was share-based payments. The large increase in share-based payments for the nine months ended September 30, 2012 compared to the nine months ended September 30, 2011 was due to increases in the recognition of the fair value of shares issued from the Del Mar Employee share Purchase Trust (“Trust”) to employees and consultants for services rendered to us, stock option expenses as our first grant of stock options occurred in February 2012, the recognition of the fair value of shares issued for services, and the increase in the fair value amount recognized for units issued for services. In the prior period shares issued from the Trust did not occur until October 2011 and there were no shares issued for services to September 30, 2011 so as a result there were no expenses related to these two items recognized during the nine months ended September 30, 2011. Units were issued for services in both periods but for the nine months ended September 30, 2012 agreements applicable to units issued for services covered the entire nine months ended September 30, 2012 while in the nine months ended September 30, 2011 units for services were applicable for two months resulting in a lower expense in the prior period.
Additionally, contracted research and travel were higher during the nine months ended September 30, 2012 compared to the nine months ended September 30, 2011. Contracted research costs were higher in the current period due to the initiation of pre-clinical research studies supporting new indications in the current period. There were no such preclinical studies on-going in the prior period. Travel has increased in the current period compared to the prior period as a result of increased travel to scientific and medical conferences. Partially offsetting the impact of higher contracted research, travel and share-based payments was a reduction in clinical development expenses related to the clinical trials being undertaken with VAL-083 for the nine months ended September 30, 2012 compared to the nine months ended September 30, 2011. The clinical development costs were lower in the current period compared to the prior largely due to clinical preparation and start-up costs incurred in the nine months ended September 30, 2011 compared to the nine months ended September 30, 2012.
General and Administrative
General and administrative expenses were $781,324 for the nine months ended September 30, 2012 compared to $127,833 for the nine months ended September 30, 2011. The principal reasons for the increase were due to higher professional fees, share-based payments, and personnel costs incurred in the current period compared to the prior period. The increase in professional fees related to costs incurred for the initiation of our first financial statement audit, legal fees related to the updating of our corporate records, and for business development fees incurred in relation to our collaboration in China and for activities relating to preparation for our financing expected to be completed in January 2013. Share-based payments have increased partially due to stock option expenses as our first grant of stock options occurred in February 2012. Additionally, units were issued for services in both periods but for the nine months ended September 30, 2012 agreements applicable to units issued for services covered the entire nine months ended September 30, 2012 while in the nine months ended September 30, 2011 units for services were applicable for two months resulting in a lower expense in the prior period. Personnel costs increased in the nine months ended September 30, 2012 compared to the nine months ended September 30, 2011 due to an increase in salaries paid in the current period compared to the prior period.
Foreign Exchange (Gain) Loss
Our functional currency is the CDN but we report our results in USD. The translation gains and losses are reported in other comprehensive loss/income. Foreign exchange gains and losses are the result of us incurring expenses in USD and then translating those USD expenses into CDN. We will continue to incur some expenses in USD and as a result will continue to be exposed to foreign exchange gains and losses in part because it is expected that the CDN will continue to be our functional currency.
We recognized a foreign exchange gain of $26,891 for the nine months ended September 30, 2012 compared to a loss of $32,965 for the nine months ended September 30, 2011. The change was due to changes in the exchange rate between the CDN and the USD and to varying levels of USD accounts payable.
Interest Expense
Pursuant to a loan agreement dated February 3, 2011, we received a loan from Valent in the amount of $250,000 for the purchase of the prototype drug product. The loan is unsecured and bears interest at 3.00% per year. As a result of the loan payable we recognized $5,630 and $5,053 respectively in accrued interest for the nine month periods ended September 30, 2012 and 2011. During the nine months ended September 30, 2011 we were charged $10,627 in interest expense relating to outstanding trade payable balances.
For the Year Ended December 31, 2011 Compared to the Period From April 6, 2010 (Inception) to December 31, 2010
Selected Annual Information
The financial information reported here in has been prepared in accordance with US GAAP. Our functional currency is CDN but we report our results in USD. The following table represents selected financial information for us as of December 31, 2011 and 2010.
Selected Balance Sheet Data
| |
|
December 31,
2011
$
|
|
|
December 31,
2010
$
|
|
| |
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
|
15,018 |
|
|
|
24,375 |
|
|
Working capital (deficiency)
|
|
|
(770,987 |
) |
|
|
(1,516 |
) |
|
Total Assets
|
|
|
68,017 |
|
|
|
299,259 |
|
|
Derivative liability
|
|
|
(106,146 |
) |
|
|
- |
|
|
Total shareholder’s deficiency
|
|
|
(877,133 |
) |
|
|
(1,516 |
) |
Liquidity and Capital Resources
| |
|
December 31,
2011
$
|
|
|
December 31,
2010
$
|
|
|
Change
$
|
|
|
Change
%
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets
|
|
|
68,017 |
|
|
|
299,259 |
|
|
|
(231,242 |
) |
|
|
(77 |
) |
|
Current liabilities
|
|
|
(839,004 |
) |
|
|
(300,775 |
) |
|
|
(538,229 |
) |
|
|
(179 |
) |
|
Working capital (deficiency)
|
|
|
(770,987 |
) |
|
|
(1,516 |
) |
|
|
(769,471 |
) |
|
|
50,757 |
|
| |
|
December 31,
2011
$
|
|
|
December 31,
2010
$
|
|
|
Change
$
|
|
|
Change
%
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash used in operating activities
|
|
|
(228,689 |
) |
|
|
(49,189 |
) |
|
|
(179,500 |
) |
|
|
(365 |
) |
|
Cash flows from financing activities
|
|
|
219,332 |
|
|
|
73,564 |
|
|
|
145,768 |
|
|
|
198 |
|
Comparison of Cash Flow
Operating Activities
Net cash used in operating activities increased to $228,689 for the year ended December 31, 2011 from $49,189 for the period ended December 31, 2010. The increase was largely the result of an increase in the net loss to $1,333,011 for the year ended December 31, 2011 compared to $108,759 for the period ended December 31, 2010. We were incorporated on April 6, 2010 and did not have significant operations until the last quarter of 2010 while in 2011 we operated for a full year. Partially offsetting the impact of the higher net loss were non-cash items totaling $536,543 incurred in the current period consisting of interest, units issued for services, warrants issued for patents, share-based compensation and the non-cash acquisition of the prototype drug product. The only non-cash item from the prior period was $32,091 for share-based payments. The most significant change in non-cash working capital for the year ended December 31, 2011 was an inflow of $596,229 from an increase in accounts payable and accrued liabilities compared to an inflow of $31,000 for the period ended December 31, 2010.
Financing Activities
We received $190,826 in net proceeds from the issuance of units and $28,506 in proceeds from the issuance of common shares during the year ended December 31, 2011 compared to $73,564 in proceeds from the issuance of common shares during the period ended December 31, 2010.
Operating Capital and Capital Expenditure Requirements
For the year ended December 31, 2011, we reported a loss of $1,333,011 and an accumulated deficit of $1,441,770 at that date. As at December 31, 2011, DelMar (BC) has cash and cash equivalents on hand of $15,018 and a negative working capital balance of $770,987. We do not have the prospect of achieving revenues in the near future and DelMar (BC) will require additional funding to maintain its research and development projects and for general operations. These circumstances lend substantial doubt as to the ability of DelMar (BC) to meet its obligations as they come due.
Consequently, management is pursuing various financing alternatives to fund DelMar (BC)’s operations so it can continue as a going concern. In addition, we have not begun to commercialize or generate revenues from any product candidate. Accordingly, we are considered to be in the development stage as defined in Accounting Standards Codification (ASC) 915-10. Management plans to secure the necessary financing through the issue of new equity and/or the entering into of strategic partnership arrangements. Nevertheless, there is no assurance that these initiatives will be successful.
Our financial statements have been prepared on a going concern basis which assumes that DelMar (BC) will continue its operations for the foreseeable future and contemplates the realization of assets and the settlement of liabilities in the normal course of business.
The conditions and risks noted above cast substantial doubt on the validity of that assumption. Our financial statements do not give effect to any adjustments to the amounts and classification of assets and liabilities that may be necessary and could potentially be material, should DelMar (BC) be unable to continue as a going concern.
Selected Statement of Operations Data
| |
|
Year Ended December 31,
2011
$
|
|
|
Period From April 6, 2010 (inception) to December 31,
2010
$
|
|
|
Change
$
|
|
|
Change
%
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
1,051,139 |
|
|
|
41,657 |
|
|
|
1,009,482 |
|
|
|
2,423 |
|
|
General and administrative
|
|
|
241,802 |
|
|
|
67,599 |
|
|
|
174,203 |
|
|
|
258 |
|
|
Foreign exchange (gain) loss
|
|
|
18,137 |
|
|
|
(497 |
) |
|
|
18,634 |
|
|
|
3,749 |
|
|
Interest expense
|
|
|
21,933 |
|
|
|
- |
|
|
|
21,933 |
|
|
|
100 |
|
|
Loss from operations
|
|
|
1,333,011 |
|
|
|
108,759 |
|
|
|
1,224,252 |
|
|
|
1,126 |
|
|
Weighted average number of shares outstanding
|
|
|
8,527,466 |
|
|
|
6,145,688 |
|
|
|
- |
|
|
|
- |
|
|
Loss per share
|
|
|
(0.16 |
) |
|
|
(0.02 |
) |
|
|
- |
|
|
|
- |
|
Year Ended December 31, 2011 compared to the Period From April 6, 2010 (Inception) to December 31, 2010
Research and Development
Research and development expenses increased to $1,051,139 for the year ended December 31, 2011 from $41,657 for the period ended December 31, 2010. We were incorporated on April 6, 2010 and for the period ended December 31, 2010 focused on corporate development and technology acquisition. The largest components of research and development for the year ended December 31, 2011 were clinical development expenses related to the clinical trials being undertaken with VAL-083, share-based payments related primarily to units issued to our management for services rendered to us, and to intellectual property costs related to our acquisition of the VAL-083 patents from Valent. It is expected that research and development costs will continue to increase in the future as we continue our clinical trials, pursue expansion of the indications for VAL-083, and look to advance our collaboration in China.
General and Administrative
General and administrative expenses were $241,802 for the year ended December 31, 2011 compared to $67,599 for the period ended December 31, 2010. In addition to the impact of us operating for a full year in 2011 compared to a partial year in 2010, general and administrative expenses increased primarily due to travel expenses to attend business development meetings and conferences and to share-based payments related primarily to units issued to our management for services rendered to us. It is expected that general and administrative expenses will increase in the future as we will require additional administrative support for its expansion of its research and development activities.
Foreign Exchange (Gain) Loss
Our functional currency is the CDN but we report our results in USD. The translation gains and losses are reported in other comprehensive loss/income. Foreign exchange gains and losses are the result of our incurring expenses in USD and then translating those USD expenses into CDN. We will continue to incur some expenses in USD and as a result will continue to be exposed to foreign exchange gains and losses in part because it is expected that the CDN will continue to be our functional currency.
We recognized a foreign exchange loss of $18,137 for the year ended December 31, 2011 compared to a gain of $497 for the period ended December 31, 2010. The change was due to changes in the exchange rate between the CDN and the USD and to varying levels of USD accounts payable.
Interest Expense
Pursuant to a loan agreement dated February 3, 2011, we received a loan from Valent in the amount of $250,000 for the purchase of the prototype drug product. The loan is unsecured and bears interest at 3.00% per year. As a result of the loan payable at December 31, 2011 we recognized $6,831 in accrued interest. During the year ended December 31, 2011 we were charged $15,102 in interest expense relating to outstanding trade payable balances. Neither of these items occurred in the period ended December 31, 2010. Interest expense on the Valent loan is expected to continue into future periods.
Off Balance Sheet Arrangements
We do not have any off balance sheet arrangements.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses and related disclosure of contingent assets and liabilities. We review our estimates on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe to be reasonable under the circumstances. Actual results may differ from these estimates. We believe the judgments and estimates required by the following accounting policies to be critical in the preparation of our financial statements.
Share for Services
We have issued equity instruments for services provided by to employees and nonemployees. The equity instruments are valued at the fair value of the instrument granted. We have transferred shares from the DelMar Employee Share Purchase Trust (the “Trust”) to consultants and management in exchange for services rendered to the Company. We recognize the fair value of the shares transferred as an expense with a corresponding increase in common stock. The shares reserved for issuance to consultants and management that are held by the Trust are included in the financial statements at year end. There are no other assets in the Trust. The shares transferred from the Trust have been valued using the fair value of the shares transferred. We have used recent share transactions in order to determine the fair value of the shares transferred from the Trust.
Stock options
We account for these awards under ASC 718, “Compensation - Stock Compensation” (“ASC 718”). ASC 718 requires measurement of compensation cost for all stock-based awards at fair value on the date of grant and recognition of compensation over the requisite service period for awards expected to vest. Compensation expense for unvested options to non-employees is revalued at each period end and is being amortized over the vesting period of the options. The determination of grant-date fair value for stock option awards is estimated using the Black-Scholes model, which includes variables such as the expected volatility of our share price, the anticipated exercise behavior of its grantee, interest rates, and dividend yields. These variables are projected based on our historical data, experience, and other factors. Changes in any of these variables could result in material adjustments to the expense recognized for share-based payments. Such value is recognized as expense over the requisite service period, net of estimated forfeitures, using the straight-line attribution method. The estimation of stock awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from current estimates, such amounts are recorded as a cumulative adjustment in the period estimates are revised. We consider many factors when estimating expected forfeitures, including type of awards granted, employee class, and historical experience. Actual results and future estimates may differ substantially from current estimates.
Derivative liability
We account for certain warrants under the authoritative guidance on accounting for derivative financial instruments indexed to, and potentially settled in, a company’s own stock, on the understanding that in compliance with applicable securities laws, the warrants require the issuance of securities upon exercise and do not sufficiently preclude an implied right to net cash settlement. We classify warrants in our balance sheet as a derivative liability which is fair valued at each reporting period subsequent to the initial issuance. We use a probability-weighted Black-Scholes pricing model to value the warrants. Determining the appropriate fair-value model and calculating the fair value of warrants requires considerable judgment. Any change in the estimates (specifically probabilities) used may cause the value to be higher or lower than that reported. The estimated volatility of our common stock at the date of issuance, and at each subsequent reporting period, is based on the historical volatility of similar life sciences companies. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the warrants at the valuation date. The expected life of the warrants is assumed to be equivalent to their remaining contractual term.
Item 3. Properties.
Our corporate headquarters are located at Suite 720-999 West Broadway, Vancouver, British Columbia, Canada. Our clinical operations are managed at 3475 Edison Way, Suite R, Menlo Park, California. Our current monthly base rent for our corporate headquarters is Cdn $1,600 under a month-to-month lease that commenced in February 2012. We also maintain access to our facilities in California through an agreement with Valent. Our leased premises, academic relationships, and access to the Valent facility are sufficient to meet the immediate needs of our business, research and operations.
Item 4. Security Ownership of Certain Beneficial Owner and Management.
The following table sets forth certain information, as of the date of filing of this report, with respect to the beneficial ownership of the outstanding common stock by (i) any holder of more than five (5%) percent; (ii) each of the Company’s executive officers and directors; and (iii) the Company’s directors and executive officers as a group. Except as otherwise indicated, each of the stockholders listed below has sole voting and investment power over the shares beneficially owned.
|
Name of Beneficial Owner (1)
|
|
Common Stock
Beneficially Owned
|
|
|
Percentage of
Common Stock (2)
|
|
|
Directors and Officers:
|
|
|
|
|
|
|
|
Jeffrey Bacha
|
|
|
6,837,083
|
(3)
|
|
|
30.7
|
%
|
|
Dennis Brown
|
|
|
3,893,542
|
(4) |
|
|
23.7
|
% |
|
Bill Garner
|
|
|
200,000
|
(5) |
|
|
1.3
|
% |
|
Lisa Guise
36 Mclean Street
Red Bank, NJ 07701
|
|
|
100,000
|
(6)
|
|
|
|
*
|
|
Scott Praill
|
|
|
150,000
|
(12) |
|
|
|
* |
|
All officers and directors as a group
|
|
|
11,180,625
|
|
|
|
47.4
|
%
|
|
Beneficial owners of more than 5%:
|
|
|
|
|
|
|
|
|
|
Howard K. Fuguet
|
|
|
2,000,000
|
(7)
|
|
|
12.2
|
%
|
|
Don Bahout
|
|
|
2,085,000
|
(8)
|
|
|
12.6
|
%
|
|
Robert Mike Newsome
|
|
|
1,152,500
|
(9)
|
|
|
7.2
|
% |
|
Raymond L. Vollintine
|
|
|
2,031,000
|
(10) |
|
|
12.3
|
% |
|
Bershaw & Co. FBO Salida Accelerator Fund s.a.r.l. #013285408
|
|
|
2,000,000
|
(11) |
|
|
11.5
|
% |
* Less than 1%
| |
(1)
|
Except as otherwise indicated, the address of each beneficial owner is c/o DelMar Pharmaceuticals, Inc., Suite 720 - 999 West Broadway, Vancouver, British Columbia, Canada V5Z 1K5.
|
| |
(2)
|
Applicable percentage ownership is based on 15,445,362 shares of common stock outstanding as of January 30, 2013, together with securities exercisable or convertible into shares of common stock within 60 days January 30, 2013 for each stockholder. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Shares of common stock that are currently exercisable or exercisable within 60 days of January 30, 2013 are deemed to be beneficially owned by the person holding such securities for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
|
| |
(3)
|
Includes 6,467,083 shares issuable upon exchange of Exchangeable Shares (including 2,608,541 shares held in trust), 150,000 shares issuable upon exercise of options, and 220,000 shares issuable upon exercise of warrants.
|
| |
(4)
|
Includes 1,650,000 shares held by Valent, 840,000 shares issuable upon exercise of warrants (including 500,000 shares issuable upon exercise of warrants held by Valent), and 150,000 shares issuable upon exercise of options.
|
| |
(5)
|
Includes 50,000 shares issuable upon exercise of warrants and 150,000 shares issuable upon exercise of options. Does not include 2,593,541 shares issuable upon exchange of Exchangeable Shares held for Mr. Garner in trust by Mr. Bacha.
|
| |
(6)
|
Includes 50,000 shares issuable upon exercise of warrants.
|
|
(7)
|
Includes 1,000,000 shares issuable upon exercise of warrants.
|
|
(8)
|
Includes 1,042,500 shares issuable upon exercise of warrants.
|
|
(9)
|
Includes 576,250 shares issuable upon exercise of warrants.
|
|
(10)
|
Includes 515,500 shares held by RL Vollintine Construction Inc. and 1,015,500 shares issuable upon exercise of warrants (including 515,500 shares issuable upon exercise of warrants held by RL Vollintone Inc.
|
|
(11)
|
Includes 1,000,000 shares issuable upon exchange of Exchangeable Shares and 1,000,000 shares issuable upon exercise of warrants.
|
|
(12)
|
Includes 50,000 shares issuable upon exercise of options.
|
Item 5. Directors and Executive Officers.
Below are the names and certain information regarding the Company’s executive officers and directors following the acquisition of DelMar (BC).
|
Name
|
|
|
Age
|
|
Position
|
| |
|
|
|
|
|
|
Jeffrey Bacha (1)
|
|
|
44
|
|
President and Chief Executive Officer
|
| |
|
|
|
|
|
|
Dennis Brown
|
|
|
63
|
|
Chief Scientific Officer
|
|
|
|
|
|
|
|
|
Scott Praill
|
|
|
46
|
|
Chief Financial Officer
|
| |
|
|
|
|
|
|
Bill Garner (1)
|
|
|
46
|
|
--
|
| |
|
|
|
|
|
|
John K. Bell (1)
|
|
|
66 |
|
--
|
| |
|
|
|
|
|
|
Lisa Guise (1)
|
|
|
42
|
|
Director
|
(1) Effective upon the Company’s meeting its information obligations under the Exchange Act Lisa Guise will resign as a director of the Company, and Jeffrey Bacha, Dennis Brown, Bill Garner and John K. Bell will be elected directors of the Company.
Jeffrey Bacha, BSc, MBA, Chief Executive Officer, President, is one of our founders and has been President, Chief Executive Officer and Director of DelMar (BC) since inception. Mr. Bacha is a seasoned executive leader with nearly twenty years of life sciences experience in the areas of operations, strategy and finance. His background includes successful public and private company building from both a start-up and turn around perspective; establishing and leading thriving management and technical teams; and raising capital in both the public and private markets. From July 2006 to August 2009, Mr. Bacha was Executive Vice President Corporate Affairs and Chief Operating Officer at Clera, Inc. From March 2005 to July 2006 Mr. Bacha was Consultant and held various positions at Clera Inc., Urigen Holdings Inc. and XBiotech, Inc. From 1999 through 2004, Mr. Bacha served as President & CEO of Inimex Pharmaceuticals, a venture-capital funded drug discovery and development company and is a former Senior Manager and Director of KPMG Health Ventures. Mr. Bacha holds an MBA from the Goizueta Business School at Emory University and a degree in BioPhysics from the University of California, San Diego.
Dr. Dennis M. Brown, PhD, Chief Scientific Officer, is one of our founders and has served as Chief Scientific Officer and Director of DelMar (BC) since inception. Dr. Brown has more than thirty years of drug discovery and development experience. He has served as Chairman of Mountain View Pharmaceutical's Board of Directors since 2000 and is the President of Valent. In 1999 he founded ChemGenex Therapeutics, which merged with a publicly traded Australian company in 2004 to become ChemGenex Pharmaceuticals (ASX: CXS/NASDAQ: CXSP), of which he served as President and a Director until 2009. He was previously a co-founder of Matrix Pharmaceutical, Inc., where he served as Vice President (VP) of Scientific Affairs from 1985-1995 and as VP, Discovery Research, from 1995-1999. He also previously served as an Assistant Professor of Radiology at Harvard University Medical School and as a Research Associate in Radiology at Stanford University Medical School. He received his B.A. in Biology and Chemistry (1971), M.S. in Cell Biology (1975) and Ph.D. in Radiation and Cancer Biology (1979), all from New York University. Dr. Brown is an inventor of about 34 issued U.S. patents and applications, many with foreign counterparts.
Scott Praill, Chief Financial Officer, has been Chief Financial Officer of the Company since January 29, 2013 and previously served as a consultant to DelMar (BC). Since 2004, Mr. Praill has been an independent consultant providing accounting and administrative services to companies in the resource industry. Mr. Praill served as CFO of Strata Oil & Gas, Inc. from June 2007 to September 2008. From November 1999 to October 2003 Mr. Praill was Director of Finance at Inflazyme Pharmaceuticals Inc. Mr. Praill completed his articling at Price Waterhouse (now PricewaterhouseCoopers LLP) and obtained his Chartered Accountant designation in 1996. Mr. Praill obtained his Certified Public Accountant (Illinois) designation in 2001. Mr. Praill received a Financial Management Diploma (Honors), from British Columbia Institute of Technology in 1993, and a Bachelor of Science from Simon Fraser University in 1989.
Dr. Bill Garner, MD, MPH. Dr. Garner is one of our founders and has served as a director of DelMar (BC) since inception and is currently CEO of Invion Ltd. (ASX:IVX). Dr. Garner is an experienced entrepreneur and investor and is a three-time Kauffman Finalist. He served as President and Chief Executive Officer of Urigen Pharmaceuticals, Inc. (URGP.PK) from December 2005 to December 2010 where he moved a procedure-based drug from a university license to a phase II multi-center clinical trial which achieved statistical significance on all end points in Painful Bladder Syndrome/Interstitial Cystitis. He is founder and managing director of EGB Advisors, LLC, a pharmaceutical commercialization boutique. Through this entity, Dr. Garner has worked on a number of pharmaceutical business transactions and has raised financing for both Urigen Pharmaceuticals, Inc. and another company that he founded, Inverseon, Inc., which is developing a novel therapy for smoking cessation, asthma and other pulmonary diseases. Before this, Dr. Garner worked in medical affairs at Hoffmann LaRoche in oncology. Prior to Roche, Dr. Garner was in the venture capital department at Paramount Capital Investments in New York City. He serves on the boards of ImmunoGenetix in Kansas City and Angel Investor Card in San Francisco. Dr. Garner has a Master of Public Health from Harvard and received his M.D. degree from New York Medical College. Dr. Garner did residency training in Anatomic Pathology at Columbia-Presbyterian and is currently a licensed physician in the State of New York.
Lisa Guise will resign as a director of the Company effective upon the Company’s meeting its information obligations under the Exchange Act. Ms. Guise served as Chief Executive Officer, Chief Financial Officer, President, Secretary, Treasurer and sole director of Berry from November 2011 until the closing of the Reverse Acquisition on January 25, 2013. Ms. Guise graduated Syracuse University. Ms. Guise received her Bachelor's of Science degree in speech communications in 1991. Over the past few years Ms. Guise has been an independent business consultant. Her experience includes working with management of privately-held companies to maximize productivity as well as general corporate matters. Ms. Guise has experience in various industries including fitness and transportation.
John K. Bell. John K. Bell is Chairman of Onbelay Capital Inc, a Canadian based private equity Company with principal investments in Telematics and auto parts manufacturing (for past 5 years). Prior to that, from 1996 to 2005, Mr. Bell was CEO and owner of Polymer Technologies Inc., an automotive parts manufacturer. Prior to that, from 1977 to 1995, Mr. Bell was founder and owner of Shred-Tech Limited a global manufacturer and supplier of industrial shredders and mobile document shredders. Mr Bell served as interim CEO and director of ATS Automation Tooling Systems (TSX-ATA) in 2007. Mr. Bell is a director of BSM Wireless (TSX-GPS), Strongco Corporation (TSX-SQP), and the Royal Canadian Mint (TSX-MNT). Mr. Bell is National secretary and board member of The Crohns and Colitis Foundation of Canada. Mr. Bell is past Chairman of Waterloo Regional Police, Cambridge Memorial Hospital, Canada’s Technology Triangle accelerator network and The Region of Waterloo prosperity counsel. Mr. Bell is a graduate of Western University Ivey School of Business, a Fellow of the institute of Chartered Accountants of Ontario, a graduate of the Institute of Directors Program of Canada and the owner’s president program at Harvard and International marketing program at Oxford.
The Company’s directors are elected at the annual meeting of shareholders to hold office until the annual meeting of shareholders for the ensuing year or until their successors have been duly elected and qualified. Officers are elected annually by the Board of Directors and serve at the discretion of the Board.
Board Leadership Structure and Role in Risk Oversight
Due to the small size and early stage of the Company, we have not adopted a formal policy on whether the Chairman and Chief Executive Officer positions should be separate or combined. Following Ms. Guise's resignation, these roles will be combined with Mr. Bacha serving as Chief Executive Officer and Chairman.
Our Board of Directors is primarily responsible for overseeing our risk management processes on behalf of the Company. The Board of Directors receives and reviews periodic reports from management, auditors, legal counsel, and others, as considered appropriate regarding our company’s assessment of risks. The Board of Directors focuses on the most significant risks facing our company and our company’s general risk management strategy, and also ensures that risks undertaken by our Company are consistent with the board’s appetite for risk. While the board oversees our company’s risk management, management is responsible for day-to-day risk management processes. We believe this division of responsibilities is the most effective approach for addressing the risks facing our company and that our board leadership structure supports this approach.
Involvement in Certain Legal Proceedings
To our knowledge, our directors and executive officers have not been involved in any of the following events during the past ten years:
| |
1.
|
any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time;
|
| |
|
|
| |
2.
|
any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses);
|
| |
|
|
| |
3.
|
being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities;
|
| |
|
|
| |
4.
|
being found by a court of competent jurisdiction in a civil action, the SEC or the Commodity Futures Trading Commission to have violated a Federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated;
|
| |
|
|
| |
5.
|
being subject of, or a party to, any Federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any Federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
|
| |
|
|
| |
6.
|
being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
|
Board Committees
Currently, the Board does not have any standing audit, nominating or compensation committees, or committees performing similar functions. The Company’s sole Director performs the duties of an audit committee. The Company’s Board does not have a nominating committee as, prior to the Reverse Acquisition, the Company had no operating business. The functions customarily performed by a nominating committee have been performed by the Company’s sole Director.
Item 6. Executive Compensation.
During its last two fiscal years, Berry did not pay any compensation to its officers or directors.
The following table sets forth all compensation paid in respect of DelMar (BC)’s principal executive officer and those individuals who received compensation in excess of $100,000 per year for 2011 and 2010. No other officer of DelMar (BC) received compensation in excess of $100,000 for 2008 and 2009.
|
Name and Principal Position
|
Year
|
|
Salary (US$)
|
|
|
Total (US$)
|
|
|
Jeffrey Bacha CEO
|
2012
|
|
$ |
144,072 |
|
|
$ |
144,072 |
|
| |
2011
|
|
$ |
60,671 |
|
|
$ |
60,671 |
|
|
Dennis Brown, Chief Scientific Officer
|
2012
|
|
$ |
120,060 |
|
|
$ |
120,060 |
|
| |
2011
|
|
$ |
60,671 |
|
|
$ |
60,671 |
|
Pursuant to consulting agreements dated August 1, 2011 with each of DelMar (BC)’s three officers and directors, DelMar (BC) agreed to compensate its officers and directors for services rendered to it, in the amount of an aggregate of Cdn $27,000 ($12,000 for Mr. Bacha, $10,000 for Dr. Brown, and $5,000 for Mr. Garner) per month commencing August 1, 2011 and ending December 31, 2012. Under the consulting agreements, DelMar (BC) and the respective officer or director mutually agreed that a portion of the compensation payable under the respective agreement for the year ended December 31, 2011 shall be deemed to have been invested in the unit offering of DelMar (BC) completed on October 3, 2011 (see “Recent Sales of Unregistered Securities”).
Under two of these agreements for the year ended December 31, 2012, the directors elected to receive a portion of their aggregate compensation in the form of units. During the nine months ended September 30, 2012 DelMar (BC) issued 360,000 units for a total amount of Cdn $180,000. The units issued relate to an amount of $15,000 per month from January to December 2012 inclusive.
The Company anticipates entering into employment agreement with Mr. Bacha and Dr. Brown in the near future.
Outstanding Equity Awards at Fiscal Year-End
Berry had no outstanding equity awards or equity compensation plan as of June 30, 2012. Effective as of the closing of the Reverse Acquisition on January 25, 2013, outstanding options to purchase 1,020,000 common shares of DelMar (BC) were deemed to be amended such that, rather than entitling the holder to acquire common shares of DelMar (BC), such options will entitle the holders to acquire shares of the Company.
Director Compensation
No director of DelMar (BC) or Berry received any compensation for services as director for DelMar (BC)’s or Berry’s last fiscal year.
Risk Management
The Company does not believe risks arising from its compensation policies and practices for its employees are reasonably likely to have a material adverse effect on the Company.
Item 7. Certain Relationships and Related Transactions, and Director Independence
Certain Relationships and Related Transactions
On September 12, 2010, DelMar (BC) entered into a Patent Assignment Agreement (the “Assignment”) with Valent Technologies LLC pursuant to which Valent assigned to DelMar (BC) its rights to patent applications and the prototype drug product related to VAL-083. In accordance with the Assignment the consideration paid by DelMar (BC) was $250,000 to acquire the prototype drug product. In accordance with the terms of the Assignment, Valent is entitled to receive a future royalty on certain revenues derived from the development and commercialization of VAL-083. In the event that DelMar (BC) terminates the agreement, DelMar (BC) may be entitled to receive royalties from Valent’s subsequent development of VAL-083 depending on the development milestones DelMar (BC) has achieved prior to the termination of the Assignment.
On January 24, 2013, the Company issued to Valent 1,150,000 shares of common stock, in exchange for Valent agreeing to reduce certain royalties payable to it under the Assignment.
Pursuant to a loan agreement dated February 3, 2011, between DelMar (BC) and Valent, Valent loaned DelMar $250,000 for the purchase of the prototype drug product under the Assignment. The loan is unsecured, bears interest at 3% per year, and is payable on demand.
In addition, under the terms of the Assignment, DelMar issued to Valent warrants to acquire 500,000 common shares at an exercise price of Cdn $0.50 per upon the completion of the financing transaction that closed in February 2012.
On April 30, 2012, DelMar (BC) issued 500,000 common shares in partial settlement of accounts payable in the amount of Cdn $250,000 (U.S. $253,050) owed to Valent.
Director Independence
Lisa Guise, our sole current director, is not independent as defined under the Nasdaq Marketplace Rules. Effective upon the Company’s meeting its information obligations under the Exchange Act, Lisa Guise will also resign as the sole director of the Company, and Jeffrey Bacha, Dennis Brown, Bill Garner, and John K. Bell will be elected directors of the Company.
Item 8. Legal Proceedings
We are not party to any legal proceedings.
Item 9. Market Price of and Dividends on Common Equity and Related Stockholder Matters
The Company’s common stock is quoted on the OTC Bulletin Board under the symbol “DMPI”. There has not been any significant trading to date in the Company’s common stock.
As of January 30, 2013, there are approximately 74 holders of record of the Company’s common stock.
As of January 30, 2013: (i) 25,746,503 shares of common stock are subject to outstanding options or warrants to purchase, or securities convertible into, common stock; (ii) 0 shares of common stock can be sold pursuant to Rule 144 under the Securities Act of 1933, as amended, and (iii) 0 shares of common stock are being, or has been publicly proposed to be, publicly offered by the Company.
Dividends
The Company has never declared or paid any cash dividends on its common stock. The Company currently intends to retain future earnings, if any, to finance the expansion of its business. As a result, the Company does not anticipate paying any cash dividends in the foreseeable future.
Securities Authorized for Issuance Under Equity Compensation Plans
As of June 30, 2012, Berry did not have any equity compensation plan. Effective as of the closing of the Reverse Acquisition on January 25, 2013, outstanding options to purchase 1,020,000 common shares of DelMar (BC) were deemed to be amended such that, rather than entitling the holder to acquire common shares of DelMar (BC), such options will entitle the holders to acquire shares of the Company.
Item 10. Recent Sales of Unregistered Securities
See Item 1.01.
On August 26, 2009, Berry issued 6,779,662 shares of common stock for an aggregate purchase price of $10,000.
Between March and May, 2010, Berry issued 10,000,000 shares of common stock for an aggregate purchase price of $29,500.
On April 29, 2010, Berry issued 3,389,821 shares of common stock for an aggregate purchase price of $10,000.
On October 24, 2011, 6,800,000 shares of commons stock of Berry were surrendered and cancelled.
On May 27, 2010, DelMar (BC) issued 7,000,000 common shares at CDN $0.001 per share for total gross proceeds of $Cdn $7,000 (U.S. $6,667).
On May 27, 2010, DelMar (BC) issued 2,000,000 common shares to the DelMar Employees Share Purchase Trust, a trust established by DelMar (BC). The shares were subsequently transferred to various consultants for services provided.
On August 27, 2010, DelMar (BC) issued 720,000 common shares at CDN $0.10 per share for total proceeds of Cdn $72,000 (U.S. $68,414).
On September 8, 2010, DelMar (BC) issued 280,000 common shares at CDN $0.10 per share for total proceeds of Cdn $28,000 (US $26,989).
DelMar (BC) issued 500,000 units on October 3, 2011, 100,000 units on October 7, 2011, and 50,000 units on November 11, 2011, at a purchase price of Cdn $0.50 per unit or total consideration of Cdn $325,000 (U.S. $310,570). Each unit consisted of one common share and share purchase warrant. Of the total consideration of Cdn $325,000, Cdn $125,000 relates to non-cash consideration received for the provision of services by officers and directors of DelMar (BC) and the settlement of accounts payable with an officer of DelMar (BC).
DelMar (BC) issued 4,150,000 units on January 23, 2012, 560,000 units on February 27, 2012, and 50,000 units on May 10, 2012, at a purchase price Cdn $0.50 per unit or total consideration of Cdn $2,380,000 (U.S.$2,365,034). The proceeds from the issuance of 3,000,000 of these units were held in escrow and subsequently the units were cancelled by DelMar (BC) and the funds returned to the subscriber.
Included in the total consideration of Cdn $2,380,000 (US $2,365,034) was Cdn $180,000 (US $181,168) relating to non-cash consideration received for the provision of services by officers and directors of DelMar (BC). All of the units issued pursuant to the consulting agreements were issued in February 2012.
Between June 1, 2012 and January 1, 2013 inclusive DelMar (BC) issued 160,000 common shares for services.
On April 30, 2012, DelMar (BC) issued 500,000 common shares in settlement of accounts payable in the amount of Cdn $250,000 (U.S. $253,050) owed to Valent.
The transactions described above were exempt from registration under Section 4(2) of the Securities Act and under Regulation S promulgated by the SEC.
Item 11. Description of Registrant’s Securities to be Registered.
The Company’s authorized capital stock consists of 200,000,000 shares of common stock, par value of $0.001 per share, and 5,000,000 shares of preferred stock, par value $0.001 per share, of which 1 share has been designated Special Voting Preferred Stock. As of the date of the filing of this report, there are 15,445,362 shares of the Company’s common stock and 1 share of Special Voting Preferred Stock issued and outstanding.
Holders of the Company’s common stock are entitled to one vote for each share on all matters submitted to a stockholder vote. Holders of common stock do not have cumulative voting rights. Therefore, holders of a majority of the shares of common stock voting for the election of directors can elect all of the directors. Holders of the Company’s common stock representing a majority of the voting power of the Company’s capital stock issued, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum at any meeting of stockholders. A vote by the holders of a majority of the Company’s outstanding shares is required to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to the Company’s certificate of incorporation.
Holders of the Company’s common stock are entitled to share in all dividends that the board of directors, in its discretion, declares from legally available funds. In the event of a liquidation, dissolution or winding up, each outstanding share entitles its holder to participate pro rata in all assets that remain after payment of liabilities and after providing for each class of stock, if any, having preference over the common stock. The Company’s common stock has no pre-emptive rights, no conversion rights and there are no redemption provisions applicable to the Company’s common stock.
The Company’s articles of incorporation authorize the issuance of 5,000,000 shares of “blank check” preferred stock, par value $0.001 per share, in one or more series, subject to any limitations prescribed by law, without further vote or action by the stockholders. Each such series of preferred stock shall have such number of shares, designations, preferences, voting powers, qualifications, and special or relative rights or privileges as shall be determined by our board of directors, which may include, among others, dividend rights, voting rights, liquidation preferences, conversion rights and preemptive rights.
Pursuant to the Certificate of Designation of the Company’s Special Voting Preferred Stock, one share of the Company’s blank check preferred stock has been designated as Special Voting Preferred Stock. The Special Voting Preferred Stock votes as a single class with the common stock and is entitled to a number of votes equal to the number of Exchangeable Shares of Exchangeco outstanding as of the applicable record date (i) that are not owned by the Company or any affiliated companies and (ii) as to which the holder has received voting instructions from the holders of such Exchangeable Shares in accordance with the Trust Agreement.
The Special Voting Preferred Stock is not entitled to receive any dividends or to receive any assets of the Company upon any liquidation, and is not convertible into common stock of the Company.
The voting rights of the Special Voting Preferred Stock will terminate pursuant to and in accordance with the Trust Agreement. The Special Voting Preferred Stock will be automatically cancelled at such time as the share of Special Voting Preferred Stock has no votes attached to it.
Item 12. Indemnification of Directors and Officers
Neither our Articles of Incorporation nor Bylaws prevent us from indemnifying our officers, directors and agents to the extent permitted under the Nevada Revised Statute ("NRS"). NRS Section 78.7502 provides that a corporation shall indemnify any director, officer, employee or agent of a corporation against expenses, including attorneys' fees, actually and reasonably incurred by him in connection with any the defense to the extent that a director, officer, employee or agent of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to Section 78.7502(1) or 78.7502(2), or in defense of any claim, issue or matter therein.
NRS 78.7502(1) provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right of the corporation, by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys' fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with the action, suit or proceeding if he: (a) is not liable pursuant to NRS 78.138; or (b) acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful.
NRS Section 78.7502(2) provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement and attorneys' fees actually and reasonably incurred by him in connection with the defense or settlement of the action or suit if he: (a) is not liable pursuant to NRS 78.138; or (b) acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation. Indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals there from, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case, the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
NRS Section 78.747 provides that except as otherwise provided by specific statute, no director or officer of a corporation is individually liable for a debt or liability of the corporation, unless the director or officer acts as the alter ego of the corporation. The court as a matter of law must determine the question of whether a director or officer acts as the alter ego of a corporation.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed hereby in the Securities Act and we will be governed by the final adjudication of such issue.
Item 13. Financial Statements and Supplementary Data
Reference is made to the filings by Berry on Form 10-K and 10-Q for Berry’s financial statements.
The financial statements of DelMar (BC) begin on Page F-1.
Item 14. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 15. Exhibits.
See Item 9.01.
Item 3.02 Unregistered Sales of Equity Securities.
See Item 1.01.
Item 4.01 Change in Registrant’s Certifying Accountant.
Effective January 25, 2013, the Board of Directors of the Company dismissed John Kinross-Kennedy (“Kinross-Kennedy”) as its independent registered accountant and engaged PricewaterhouseCoopers LLP (“PWC”) to serve as its independent registered accounting firm. Kinross-Kennedy’s audit reports on the Company’s financial statements for the fiscal years ended June 30, 2012 and 2011 did not contain an adverse opinion or a disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope or accounting principles, except that, the audit reports included an explanatory paragraph with respect to the uncertainty as to the Company’s ability to continue as a going concern. During the years ended June 30, 2012 and 2011 and during the subsequent interim period preceding the date of Kinross-Kennedy’s dismissal, there were (i) no disagreements with Kinross-Kennedy on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, and (ii) no reportable events (as that term is defined in Item 304(a)(1)(v) of Regulation S-K).
PWC is the independent registered accounting firm for DelMar (BC), and its report on the financial statements of DelMar (BC) at December 31, 2011 and 2010 and for the period from April 6, 2010 (date of incorporation) to December 31, 2010 and the year ended December 31, 2011 is included in this current report on Form 8-K. Prior to engaging PWC, the Company did not consult with PWC regarding the application of accounting principles to a specific completed or contemplated transaction, or the type of audit opinion that might be rendered on the Company’s financial statements.
The Company has requested Kinross-Kennedy to furnish it with a letter addressed to the SEC stating whether it agrees with the statements made above by the Company. The Company has filed this letter as an exhibit to this 8-K.
Item 5.01 Changes in Control of Registrant.
See Item 2.01.
Item 5.02 Departure of Directors or Principal Officers; Election of Directors; Appointment of Principal Officers.
See Item 1.01.
Item 5.03 Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year.
Effective January 25, 2013, the Company changed its fiscal year from June 30 to that of DelMar (BC) of December 31.
Item 5.06 Change in Shell Company Status.
See Item 1.01.
Item 9.01 Financial Statements and Exhibits.
(a) Financial statements of DelMar (BC) are included following the signature page.
(b) Pro forma financial information. See exhibit 99.1.
(c) Shell Company Transactions. See (a) and (b) of this Item 9.01.
|
Exhibit Number
|
|
Description
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
† Confidential treatment is requested for certain confidential portions of this exhibit pursuant to Rule 24b-2 under the Exchange Act. In accordance with Rule 24b-2, these confidential portions have been omitted from this exhibit and filed separately with the Commission
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
| |
DELMAR PHARMACEUTICALS, INC.
|
|
| |
|
|
|
|
Dated: January 31, 2013
|
By:
|
/s/ Jeffrey Bacha
|
|
| |
|
Jeffrey Bacha
|
|
| |
|
Chief Executive Officer
|
|
| |
|
|
|
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Condensed Interim Financial Statements
(Unaudited)
For the nine months ended September 30, 2012
(expressed in US dollars unless otherwise noted)
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Condensed Interim Balance Sheets
(expressed in US dollars unless otherwise noted)
| |
|
Note
|
|
|
September 30,
|
|
|
December 31,
|
|
| |
|
|
|
|
|
2012 |
|
|
|
2011 |
|
| |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
|
|
|
|
55,300 |
|
|
|
15,018 |
|
|
Taxes and other receivables
|
|
3 |
|
|
|
12,876 |
|
|
|
38,802 |
|
|
Prepaid expenses
|
|
5 |
|
|
|
91,115 |
|
|
|
14,197 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
159,291 |
|
|
|
68,017 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities
|
|
|
|
|
|
446,473 |
|
|
|
561,145 |
|
|
Loan payable
|
|
4 |
|
|
|
262,461 |
|
|
|
256,831 |
|
|
Related party payables
|
|
5 |
|
|
|
55,312 |
|
|
|
21,028 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
764,246 |
|
|
|
839,004 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Derivative liability
|
|
6 |
|
|
|
354,662 |
|
|
|
106,146 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
1,118,908 |
|
|
|
945,150 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Deficiency
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Common stock
|
|
|
|
|
|
|
|
|
|
|
|
|
Authorized - unlimited number with no par value
|
|
|
|
|
|
|
|
|
|
|
|
|
Issued and outstanding - 12,990,000 at September 30, 2012 (December 31, 2011 - 9,059,375)
|
|
7 |
|
|
|
1,936,247 |
|
|
|
418,611 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
|
|
|
207,406 |
|
|
|
103,727 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Warrants
|
|
7 |
|
|
|
313,924 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Deficit accumulated during the development stage
|
|
7 |
|
|
|
(3,418,854 |
) |
|
|
(1,441,770 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income
|
|
|
|
|
|
1,660 |
|
|
|
42,299 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
(959,617 |
) |
|
|
(877,133 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
159,291 |
|
|
|
68,017 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Nature of operations and going concern (note 1)
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Subsequent events (note 8)
|
|
|
|
|
|
|
|
|
|
|
|
Approved by the Board of Directors
| (signed) Jeffrey Bacha |
Director |
(signed) Dennis Brown |
Director |
| |
|
|
|
The accompanying notes are an integral part of these condensed interim financial statements.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Condensed Interim Statements of Loss and Comprehensive Loss
(expressed in US dollars unless otherwise noted)
| |
|
|
Three months ended
September 30,
|
|
|
Nine months ended
September 30,
|
|
|
Balance from
April 6, 2010
(inception) to
September 30,
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Notes
|
|
|
2012 |
|
|
|
2011 |
|
|
|
2012 |
|
|
|
2011 |
|
|
|
2012 |
|
| |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
|
229,488 |
|
|
|
272,462 |
|
|
|
1,217,021 |
|
|
|
620,469 |
|
|
|
2,309,817 |
|
|
General and administrative
|
|
|
|
218,732 |
|
|
|
61,279 |
|
|
|
781,324 |
|
|
|
127,833 |
|
|
|
1,090,781 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
(448,220 |
) |
|
|
(333,741 |
) |
|
|
(1,998,345 |
) |
|
|
(748,302 |
) |
|
|
(3,400,598 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (loss)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange gain (loss)
|
|
|
|
22,295 |
|
|
|
(41,738 |
) |
|
|
26,891 |
|
|
|
(32,965 |
) |
|
|
9,251 |
|
|
Interest expense
|
|
|
|
(1,900 |
) |
|
|
(10,628 |
) |
|
|
(5,630 |
) |
|
|
(15,680 |
) |
|
|
(27,507 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
20,395 |
|
|
|
(52,366 |
) |
|
|
21,261 |
|
|
|
(48,645 |
) |
|
|
(18,256 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the period
|
|
|
|
(427,825 |
) |
|
|
(386,107 |
) |
|
|
(1,977,084 |
) |
|
|
(796,947 |
) |
|
|
(3,418,854 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share
|
|
|
|
(0.03 |
) |
|
|
(0.05 |
) |
|
|
(0.15 |
) |
|
|
(0.10 |
) |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares
|
|
|
|
12,969,783 |
|
|
|
8,187,500 |
|
|
|
13,287,835 |
|
|
|
8,195,307 |
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
|
(427,825 |
) |
|
|
(386,107 |
) |
|
|
(1,977,084 |
) |
|
|
(796,947 |
) |
|
|
(3,418,854 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive (loss) income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Translation to US dollar presentation currency
|
|
|
|
(24,982 |
) |
|
|
55,632 |
|
|
|
(40,639 |
) |
|
|
53,935 |
|
|
|
1,660 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss
|
|
|
|
(452,807 |
) |
|
|
(330,475 |
) |
|
|
(2,017,723 |
) |
|
|
(743,012 |
) |
|
|
(3,417,194 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed interim financial statements.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Condensed Interim Statements of Cash Flows
(expressed in US dollars unless otherwise noted)
| |
|
Nine months ended
September 30,
|
|
|
Balance from
April 6, 2010
(inception) to
September 30,
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
2012 |
|
|
|
2011 |
|
|
|
2012 |
|
| |
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss for the period
|
|
|
(1,977,084 |
) |
|
|
(796,947 |
) |
|
|
(3,418,854 |
) |
|
Items not affecting cash
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest
|
|
|
5,630 |
|
|
|
5,053 |
|
|
|
12,461 |
|
|
Units issued for services
|
|
|
135,108 |
|
|
|
- |
|
|
|
230,248 |
|
|
Shares issued for services
|
|
|
39,626 |
|
|
|
- |
|
|
|
39,626 |
|
|
Warrants issued for patents
|
|
|
- |
|
|
|
- |
|
|
|
89,432 |
|
|
Warrants issued for services
|
|
|
49,379 |
|
|
|
- |
|
|
|
49,379 |
|
|
Share-based compensation
|
|
|
989,253 |
|
|
|
- |
|
|
|
1,116,484 |
|
|
Prototype drug product
|
|
|
- |
|
|
|
190,690 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
(758,088 |
) |
|
|
(601,204 |
) |
|
|
(1,881,224 |
) |
|
Changes in non-cash working capital
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other receivables
|
|
|
25,926 |
|
|
|
(750 |
) |
|
|
(12,876 |
) |
|
Prepaid expenses
|
|
|
(31,149 |
) |
|
|
(1,068 |
) |
|
|
(45,346 |
) |
|
Accounts payable and accrued liabilities
|
|
|
97,739 |
|
|
|
478,643 |
|
|
|
977,955 |
|
|
Related party payables
|
|
|
34,284 |
|
|
|
58,964 |
|
|
|
55,312 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
(631,288 |
) |
|
|
(65,415 |
) |
|
|
(906,179 |
) |
|
Cash flows from investing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net proceeds from the issuance of units
|
|
|
671,570 |
|
|
|
- |
|
|
|
859,409 |
|
|
Net proceeds from the issuance of common shares
|
|
|
- |
|
|
|
100,056 |
|
|
|
102,070 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
671,570 |
|
|
|
100,056 |
|
|
|
961,479 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase in cash and cash equivalents
|
|
|
40,282 |
|
|
|
34,641 |
|
|
|
55,300 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents - beginning of period
|
|
|
15,018 |
|
|
|
24,375 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents - end of period
|
|
|
55,300 |
|
|
|
59,016 |
|
|
|
55,300 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplementary information
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of shares for the settlement of accounts payable (notes 4 and 5)
|
|
|
253,050 |
|
|
|
- |
|
|
|
253,050 |
|
|
Issuance of units for the settlement of accounts payable
|
|
|
- |
|
|
|
- |
|
|
|
23,785 |
|
|
Non-cash share issuance costs (note 7)
|
|
|
160,818 |
|
|
|
- |
|
|
|
175,113 |
|
|
Settlement of accounts payable with a loan payable (note 4)
|
|
|
- |
|
|
|
250,000 |
|
|
|
250,000 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed interim financial statements.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
|
1
|
Going concern and nature of operations
|
Going concern
For the year nine months ended September 30, 2012, the Company reported a loss of $1,977,084 and an accumulated deficit of $3,418,854 at that date. As at September 30, 2012, DelMar had cash and cash equivalents on hand of $55,300 and a negative working capital balance of $604,955. DelMar does not have the prospect of achieving revenues in the near future and DelMar will require additional funding to maintain its research and development projects and for general operations. These circumstances lend substantial doubt as to the ability of DelMar to meet its obligations as they come due.
Consequently, management is pursuing various financing alternatives to fund DelMar’s operations so it can continue as a going concern. In addition, the Company has not begun to commercialize or generate revenues from any product candidate. Accordingly, the Company is considered to be in the development stage as defined in Accounting Standards Codification (ASC) 915-10. Management plans to secure the necessary financing through the issue of new equity and/or the entering into of strategic partnership arrangements. Nevertheless, there is no assurance that these initiatives will be successful (note 8).
These financial statements have been prepared on a going concern basis which assumes that DelMar will continue its operations for the foreseeable future and contemplates the realization of assets and the settlement of liabilities in the normal course of business.
The conditions and risks noted above cast substantial doubt on the validity of that assumption. These financial statements do not give effect to any adjustments to the amounts and classification of assets and liabilities that may be necessary and could potentially be material, should DelMar be unable to continue as a going concern.
Nature of operations
DelMar Pharmaceuticals Ltd. (“DelMar” or the “Company”) is a development stage company focused on the discovery and development of new medicines with the potential to treat cancer patients who have failed modern targeted or biologic therapy. DelMar has initiated a clinical trial with its lead drug candidate VAL-083 for the treatment of refractory glioblastoma multiforme (GBM). The Phase I/II study is an open-label, single arm dose-escalation study designed to evaluate the safety, tolerability, pharmacokinetics and anti-tumor activity of VAL-083 in patients with histologically confirmed initial diagnosis of primary WHO Grade IV malignant glioma (GBM), now recurrent. Patients with prior low-grade glioma or anaplastic glioma are eligible, if histologic assessment demonstrates transformation to GBM.
The Company efforts have been devoted to research and development, raising capital, recruitment of personnel and long-term planning. The Company is currently a private company. The Company was incorporated on April 6, 2010 under the British Columbia Business Corporations Act and is domiciled in British Columbia, Canada. The address of its registered office is Suite 720 - 999 West Broadway, Vancouver, British Columbia, V5Z 1K5.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
On May 27, 2010 the company issued shares to the founders of DelMar. As of this date, the Company did not have any operations or assets. Accordingly these founders’ shares were issued at nominal value.
In the summer of 2010 the company began discussions with Valent Technologies LLC (“Valent”) regarding the acquisition of certain intellectual property and a prototype drug product, VAL-083. During this time the company also began to develop a business plan for the development of VAL-083 as a potential new cancer therapy.
On September 12, 2010 DelMar executed a Patent Assignment Agreement with Valent to acquire the prototype drug product and certain intellectual property.
On October 20, 2010 DelMar filed an Investigational New Drug Application (“IND”) with the United States Food & Drug Administration (“FDA”) to initiate clinical trials with VAL-083 as a potential cancer treatment.
During the remainder of 2010 and the first half of 2011, DelMar conducted research requested by the FDA focused on developing new analytical methods related to manufacturing and conducting pre-clinical toxicology studies to enable the allowance of the IND. New patent applications were filed by DelMar to protect this new intellectual property.
In September 2011 DelMar announced that its IND application had been allowed by the FDA and in October, 2011 DelMar commenced its clinical trials in the United States with its lead drug candidate, VAL-083. Also in the last quarter of 2011 DelMar initiated its preclinical research into the molecular mechanism of action of VAL-083.
The prototype drug product acquired from Valent was used in DelMar’s clinical trials undertaken in 2011 and preclinical studies conducted in 2011 and 2012.
In February 2012 DelMar received approval from the FDA Office of Orphan Products Development granting orphan drug designation for VAL-083 for the treatment of glioma, including glioblastoma multiforme (“GBM”), the most common and aggressive form of brain cancer.
In April 2012 DelMar presented results of research conducted in collaboration with the University of British Columbia. These data gathered differentiated the mechanism of action of VAL-083 from other drugs approved to treat GBM.
In the second quarter of 2012 patents from Valent were assigned to DelMar and DelMar continued to file new patents for various matters linked to Val-083.
In October 2012 DelMar announced a strategic collaboration with Guangxi Wuzhou Pharmaceutical Company, a subsidiary of publicly traded Guangxi Wuzhou Zhongheng Group Co., Ltd for the development of VAL-083, known as “DAG for Injection” in China.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
In November 2012, DelMar presented interim clinical data demonstrating activity against GBM.
In January 2013 DelMar announced that the European Committee for Orphan Medicinal Products (COMP) has recommended the designation of VAL-083 as an orphan medicinal product for the treatment of glioma.
|
2
|
Significant accounting policies
|
Basis of presentation
The financial statements of DelMar have been prepared in accordance with United States Generally Accepted Accounting Principles (“US GAAP”) and are presented in United States dollars. The Company’s functional currency is the Canadian dollar.
The principal accounting policies applied in the preparation of these financial statements are set out below and have been consistently applied to all periods presented.
Unaudited interim financial data
The accompanying unaudited September 30, 2012 balance sheet, the statements of operations and comprehensive income (loss) and cash flows for the nine months ended September 30, 2011 and 2012, and the related interim information contained within the notes to the financial statements have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission for interim financial information. Accordingly, they do not include all of the information and the notes required by accounting principles generally accepted in the United States (“GAAP”) for complete financial statements. In the opinion of management, the unaudited interim financial statements reflect all adjustments, consisting of normal and recurring adjustments, necessary for the fair statement of the Company’s financial position at September 30, 2012 and results of its operations and its cash flows for the nine months ended September 30, 2011 and 2012. The results for the nine months ended September 30, 2012 are not necessarily indicative of the results to be expected for the year ending December 31, 2012 or for any other future annual or interim period.
Clinical trial expenses
Clinical trial expenses are a component of research and development costs and include fees paid to contract research organizations, investigators and other vendors who conduct certain product development activities on behalf of the Company. The amount of clinical trial expenses recognized in a period related to service agreements are based on estimates of the work performed using an accrual basis of accounting. These estimates are based on patient enrollment, services provided and goods delivered, contractual terms and experience with similar contracts. The Company monitors these factors to the extent possible and adjusts our estimates accordingly. Prepaid expenses or accrued liabilities are adjusted if payments to service providers differ from estimates of the amount of service completed in a given period.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
Share for services
The Company has issued equity instruments for services provided by to employees and nonemployees. The equity instruments are valued at the fair value of the instrument granted (see notes 6 and 7 for assumptions).
The Company has transferred shares from the DelMar Employee Share Purchase Trust (the “Trust”) to consultants and management in exchange for services rendered to the Company. The Company recognizes the fair value of the shares transferred as an expense with a corresponding increase in common stock. The shares reserved for issuance to consultants and management that are held by the Trust are included in the financial statements at year end. There are no other assets in the Trust. The number of shares outstanding for issue from the Trust at September 30, 2012 is nil (December 31, 2011 - 1,590,625) (note 7).
The shares transferred from the Trust have been valued using the fair value of the shares transferred. The Company has used recent share transactions in order to determine the fair value of the shares transferred from the Trust.
Stock options
The Company accounts for these awards under ASC 718, “Compensation - Stock Compensation” (“ASC 718”). ASC 718 requires measurement of compensation cost for all stock-based awards at fair value on the date of grant and recognition of compensation over the requisite service period for awards expected to vest. Compensation expense for unvested options to non-employees is revalued at each period end and is being amortized over the vesting period of the options. The determination of grant-date fair value for stock option awards is estimated using the Black-Scholes model, which includes variables such as the expected volatility of the Company’s share price, the anticipated exercise behavior of its grantee, interest rates, and dividend yields. These variables are projected based on the Company’s historical data, experience, and other factors. Changes in any of these variables could result in material adjustments to the expense recognized for share-based payments. Such value is recognized as expense over the requisite service period, net of estimated forfeitures, using the straight-line attribution method. The estimation of stock awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from current estimates, such amounts are recorded as a cumulative adjustment in the period estimates are revised. The Company considers many factors when estimating expected forfeitures, including type of awards granted, employee class, and historical experience. Actual results and future estimates may differ substantially from current estimates.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
Derivative liability
The Company accounts for certain warrants under the authoritative guidance on accounting for derivative financial instruments indexed to, and potentially settled in, a company’s own stock, on the understanding that in compliance with applicable securities laws, the warrants require the issuance of securities upon exercise and do not sufficiently preclude an implied right to net cash settlement. The Company classifies warrants in its balance sheet as a derivative liability which is fair valued at each reporting period subsequent to the initial issuance. The Company uses a probability-weighted Black-Scholes pricing model to value the warrants. Determining the appropriate fair-value model and calculating the fair value of warrants requires considerable judgment. Any change in the estimates (specifically probabilities) used may cause the value to be higher or lower than that reported. The estimated volatility of the Company’s common stock at the date of issuance, and at each subsequent reporting period, is based on the historical volatility of similar life sciences companies. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the warrants at the valuation date. The expected life of the warrants is assumed to be equivalent to their remaining contractual term.
Loss per share
Income or loss per share is calculated based on the weighted average number of common shares outstanding. Diluted loss per share does not differ from basic loss per share since the effect of the Company’s warrants is anti-dilutive. Diluted income per share is calculated using the treasury stock method which uses the weighted average number of common shares outstanding during the period and also includes the dilutive effect of potentially issuable common shares from outstanding stock options and warrants. At September 31, 2012, potential common shares of 3,360,000 (September 30, 2011 - nil) relating to warrants and 1,020,000 (September 30, 2011 - nil) relating to stock options were excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive.
Segment information
The Company identifies its operating segments based on business activities, management responsibility and geographical location. The Company operates within a single operating segment being the research and development of cancer indications, and operates in one geographic area, being Canada. All of the Company’s assets are located in Canada.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
Recent accounting pronouncements
The Company reviews new accounting standards as issued. No new standards had any material effect on these financial statements. The accounting pronouncements issued subsequent to the date of these financial statements that were considered significant by management were evaluated for the potential effect on these financial statements. Management does not believe any of the subsequent pronouncements will have a material effect on these financial statements as presented and does not anticipate the need for any future restatement of these financial statements because of the retro-active application of any accounting pronouncements issued subsequent to September 30, 2012 through the date these financial statements were issued.
|
3
|
Taxes and other receivables
|
On May 1, 2012 the Company was granted a third non-repayable financial contribution of up to $48,820 (CDN $48,000) from the National Research Council of Canada Industrial Research Assistance Program (“IRAP”). Awards under the IRAP grant directly reduce the Company’s research and development costs by 75% of eligible expenses. Total expenses under this program will be $48,820 (CDN $48,000) of which $36,615 (CDN $36,000) will be reimbursed through IRAP. Under this IRAP grant the Company requested an aggregate total reimbursement of $10,209 (CDN $10,038) and has received $2,267 (CDN $2,583) to September 30, 2012 resulting in a receivable of $7,942 (CDN $7,455) at September 30, 2012.
|
4
|
Valent Technologies LLC agreement
|
Pursuant to a loan agreement dated February 3, 2011, the Company has entered a loan with Valent Technologies LLC (“Valent”) for $250,000 for the purchase of the prototype drug product. The loan is unsecured and bears interest at 3.00% per year. The loan payable balance at September 30, 2012 is $262,461. The Company has accrued interest of $5,630 for the nine months ended September 30, 2012 (September 30, 2011 - $5,053).
Pursuant to its agreement with Valent, the Company is required to issue warrants to Valent under certain circumstances. The financing completed by the Company that closed in February 2012 resulted in the Company issuing 500,000 warrants to Valent on February 1, 2012 at an exercise price of CDN $0.50 per warrant (note 5). In exchange for the warrants Valent has assigned all of its right, title and interest in and to the patents for VAL-083 to the Company. The fair value of the contingent warrants of $89,432 has been recognized as an expense and a corresponding increase to additional paid-in capital at December 31, 2011. As a result of the warrants being issued the amount previously recognized as additional paid in capital has been reclassified to warrants at September 30, 2012.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
|
5
|
Related party transactions
|
During the nine months ended September 30, 2012
Pursuant to consulting agreements with the Company’s three directors the Company pays a total of CDN $27,000 per month to its directors. Under two of these agreements the directors have elected to receive a portion of their aggregate compensation in the form of units. During the nine months ended September 30, 2012 the Company issued 360,000 units for a total amount of $181,168. The units issued relate to an amount of CDN $15,000 per month from January to December 2012 inclusive. All of the units were issued in February 2012 so as a result, the Company has recognized $135,108 in services and $46,060 in prepaid consulting at September 30, 2012 (note 6). Of the $135,108, $46,060 has been recognized as general and administrative and $89,048 has been recognized as research and development.
Additionally, under the consulting agreements the Company has paid two of its directors cash compensation totaling an aggregate $11,494 (CDN $12,000) per month. An amount of $103,447 (CDN $108,000) has been paid to the two directors for the nine months ended September 30, 2012.
Included in accounts payable at September 30, 2012 is an aggregate amount owing of $55,312 to two of the Company’s directors.
Also included in accounts payable at September 30, 2012 is an amount of $244,007 relating to clinical development costs incurred by Valent on behalf of the Company. On April 30, 2012, Valent was issued 500,000 common shares for partial settlement of the Company’s accounts payable balance with Valent. The total settlement amount was $253,050. Additionally, the Company also has a loan payable, including accrued interest, of $262,461 due to Valent at September 30, 2012. One of the directors and officers of the Company is also a Principal of Valent.
The Company granted an aggregate of 450,000 stock options at an exercise price of CDN $0.50 to its three directors (note 7).
The Company transferred a total of 1,390,625 shares from the DelMar Employee Share Purchase Trust in three equal tranches to each of the Company’s three directors (note 7).
During the nine months ended September 30, 2011
Pursuant to the consulting agreements with each of the Company’s officers and directors the Company has recognized $40,184 in compensation expense. Of the $40,184, $10,046 has been recognized as general and administrative and $30,138 has been recognized as research and development.
Additionally, the Company has paid $54,248 in cash compensation to one of its officers.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
The Company issued 4,150,000 units on January 23, 2012, 560,000 on February 27, 2012, and 50,000 on May 10, 2012. The total 4,760,000 units were issued for CDN $0.50 per unit or total consideration of $2,365,034 (CDN $2,380,000). The proceeds from the issuance of 3,000,000 of these units were held in escrow and subsequently the units were cancelled by the Company and the funds returned to the subscriber. Of the remaining 1,760,000 units, 460,000 of the share purchase warrants issued expire October 31, 2013 and 1,300,000 expire on December 31, 2013. Depending on certain circumstances, the warrants have an escalating exercise price and a cashless exercise provision.
Included in the total consideration of $2,365,034 was $181,168 relating to non-cash consideration received for the provision of services by officers and directors of the Company (note 5). All of the units issued pursuant to the consulting agreements were issued in February 2012
In September 2011 the Company received $71,550 in proceeds relating to units issued in October 2011. As a result, the Company has recognized a subscriptions payable of $71,550 at September 30, 2011.
Common stock
Authorized
Unlimited common shares without par value
Issued and outstanding at September 30, 2012 - 12,990,000 (December 31, 2011 - 9,059,375)
| |
|
Number of shares
|
|
|
Amount
$
|
|
| |
|
|
|
|
|
|
|
Balance – December 31, 2011
|
|
|
9,059,375 |
|
|
|
418,611 |
|
| |
|
|
|
|
|
|
|
|
|
Proceeds allocated from the issuance of units (note 6)
|
|
|
4,760,000 |
|
|
|
1,514,804 |
|
|
Units subsequently cancelled (note 6)
|
|
|
(3,000,000 |
) |
|
|
(941,813 |
) |
|
Shares issued for services (a)
|
|
|
80,000 |
|
|
|
39,626 |
|
|
Fair value of shares issued from the Del Mar Employee Share Purchase Trust for services (b)
|
|
|
1,590,625 |
|
|
|
781,846 |
|
|
Shares issued for the settlement of accounts payable (note 5)
|
|
|
500,000 |
|
|
|
253,050 |
|
|
Issue costs
|
|
|
- |
|
|
|
(129,877 |
) |
| |
|
|
|
|
|
|
|
|
|
Balance – September 30, 2012
|
|
|
12,990,000 |
|
|
|
1,936,247 |
|
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
|
a)
|
Shares issued for services
|
Pursuant to a consulting agreement dated May 1, 2012 the Company is required to issue 20,000 common shares a month from June 1, 2012 to May 1, 2013 inclusive. Under this agreement the Company has issued 80,000 shares to September 30, 2012. The shares have been valued using the fair value of shares recently issued by the Company.
|
b)
|
Shares issued to the DelMar Employees Share Purchase Trust
|
| |
|
Number of
shares held
in Trust
|
|
| |
|
|
|
|
Balance – December 31, 2011
|
|
|
1,590,625 |
|
|
Shares transferred to employees and consultants for services
|
|
|
(1,590,625 |
) |
| |
|
|
|
|
|
Balance – September 30, 2012
|
|
|
- |
|
The Company has transferred shares from the Trust to various consultants for work or services performed for the Company. Of the 1,590,625 shares transferred out of the trust, an aggregate 1,390,625 were transferred in equal tranches to each of the Company’s three directors. The Company has recognized the fair value of the shares transferred resulting in the recognition of $781,846 in expense and capital stock at September 30, 2012.
Stock Options
On February 1, 2012 the Company’s board of directors approved its stock option plan (the “Plan”). Under the Plan the number of common shares that will be reserved for issuance to officers, directors, employees and consultants under the Plan will not exceed 7.5% of the share capital of the Company on a fully diluted basis. On February 1, 2012 the Company granted 930,000 options and on June 15, 2012 an additional 90,000 options were granted under the Plan. All of the stock options granted have an exercise price of CDN $0.50 and expire 10 years from the date of grant. Of the 1,020,000 stock options granted, 450,000 vest in equal monthly installments over one year and 570,000 vest in equal monthly installments over three years. Included in the total number of stock options granted were 450,000 granted in equal tranches to the Company’s three directors.
In the event of the sale of 66 2/3% of the equity securities of the Company where equity securities include shares, warrants, stock options, and any convertible securities of the Company, any options not yet granted under the Plan shall be deemed granted to the principle founders of the Company on a pro-rata basis in accordance with their ownership of the Company on a fully-diluted basis immediately prior to the closing of such a sale.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
The following table sets forth the options outstanding under the Plan as of September 30, 2012:
| |
|
Number of
stock
options
outstanding
|
|
|
Weighted
average
exercise
price
$Cdn
|
|
| |
|
|
|
|
|
|
|
Balance - December 31, 2011
|
|
|
- |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
Granted
|
|
|
1,020,000 |
|
|
|
0.50 |
|
| |
|
|
|
|
|
|
|
|
|
Balance - September 30, 2012
|
|
|
1,020,000 |
|
|
|
0.50 |
|
The following table summarizes stock options currently outstanding and exercisable at September 30, 2012:
|
Exercise price $Cdn
|
|
|
Number
outstanding at
September 30,
2012
|
|
|
Weighted
average
remaining
contractual
life
(years)
|
|
|
Weighted
average
exercise
price
$Cdn
|
|
|
Number
exercisable
at
September 30,
2012
|
|
|
Exercise
price
$Cdn
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| $ |
0.50 |
|
|
|
1,020,000 |
|
|
|
9.37 |
|
|
|
0.50 |
|
|
|
413,722 |
|
|
|
0.50 |
|
The stock options have been valued using a Black-Scholes pricing model using the following assumptions: dividend rate - 0%, volatility - 97.3%, risk free rate - 1.25% and an initial term of approximately 3 years.
The Company has recognized the following amounts as stock-based compensation expense for the periods noted:
| |
|
Three months ended
September 30,
|
|
|
Nine months ended
September 30,
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
2012 |
|
|
|
2011 |
|
|
|
2012 |
|
|
|
2011 |
|
| |
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
Research and development
|
|
|
34,907 |
|
|
|
- |
|
|
|
146,162 |
|
|
|
- |
|
|
General and administrative
|
|
|
12,180 |
|
|
|
- |
|
|
|
61,245 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
47,087 |
|
|
|
- |
|
|
|
207,407 |
|
|
|
- |
|
The aggregate intrinsic value of stock options outstanding at September 30, 2012 was $nil and the aggregate intrinsic value of stock options exercisable at September 30, 2012 was also $nil. As of September 30, 2012 there was $90,018 in unrecognized compensation expense that will be recognized over the next twenty-eight months. No stock options have been exercised under the Plan.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
A summary of status of the Company’s unvested stock options as of September 30, 2012 under all plans is presented below:
| |
|
Number of
options
|
|
|
Weighted
average
exercise
price
$Cdn
|
|
|
Weighted
average
grant date
fair value
$Cdn
|
|
| |
|
|
|
|
|
|
|
|
|
|
Unvested at December 31, 2011
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Granted
|
|
|
1,020,000 |
|
|
|
0.50 |
|
|
|
0.304 |
|
|
Vested
|
|
|
(413,722 |
) |
|
|
0.50 |
|
|
|
0.304 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unvested at September 30, 2012
|
|
|
606,278 |
|
|
|
0.50 |
|
|
|
0.304 |
|
Warrants
| |
|
Number of
Warrants
|
|
|
Amount
$
|
|
| |
|
|
|
|
|
|
|
Balance - December 31, 2011
|
|
|
- |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
Warrants issued for patents (i)
|
|
|
500,000 |
|
|
|
89,432 |
|
|
Warrants issued as unit issue costs (ii)
|
|
|
105,000 |
|
|
|
175,113 |
|
|
Warrants issued for services (iii)
|
|
|
345,000 |
|
|
|
49,379 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
950,000 |
|
|
|
313,924 |
|
|
i)
|
At December 31, 2011 the Company has recognized the fair value of the contingent Valent warrants (note 4). The contingent warrants were recognized in additional paid in capital at December 31, 2011 and have been reclassified to warrants when the warrants were issued on February 1, 2012. The fair value of the warrants was based on the fair value of the warrants included as part of the unit issuance.
|
|
ii)
|
The Company has issued broker warrants as finder’s fees in relation to the issuance of certain units. A portion of the units to which the finder’s fees relate were issued during the year ended December 31, 2011 and a portion were issued during the nine months ended September 31, 2012. A total amount of $175,113 has been recognized as warrants with $14,295 being reclassified from additional paid in capital outstanding at December 31, 2011 and $160,818 being recognized for warrants issued in the nine months ended September 30, 2012. The warrants relating to these amounts were all issued on March 1, 2012. The fair value of the warrants was based on the fair value of the warrants included as part of the unit issuance.
|
|
iii)
|
The Company has issued 345,000 warrants in for investor relations services. The warrants were issued on February 1, 2012 and they vest in 12 equal installments over a 12-month period commencing on March 1, 2012. The warrants expire on February 1, 2015. The fair value of the warrants was based on the fair value of the warrants included as part of the unit issuance.
|
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
Reverse acquisition
On January 25, 2013, the Company entered into and closed an Exchange Agreement with DelMar Pharmaceuticals Inc. (formerly Berry Only Inc. (“Berry”)) (the “Acquisition”). The Acquisition transaction will result in Berry acquiring DelMar by issuing a sufficient number of shares such that the shareholders of DelMar will have a controlling interest in Berry subsequent to the completion of the Acquisition transaction. Simultaneous with the Acquisition transaction, Valent will be issued 1,150,000 common shares of Berry. The shares issued to Valent by Berry are being issued in exchange for Valent reducing certain royalties under its agreement with DelMar. Upon completion of the Acquisition DelMar will become a wholly-owned subsidiary of Berry. As a result of the shareholders of DelMar having a controlling interest in Berry subsequent to the transaction, for accounting purposes the transaction constitutes a reverse recapitalization with DelMar being the accounting acquirer even though legally DelMar is the acquiree. Therefore, the net assets of Berry are recorded at fair value at the date of the transaction. No goodwill is recorded with respect to the transaction as it does not constitute a business combination.
Unit offering
In connection with the Exchange Agreement, on January 25, 2013 Berry entered into and closed a series of subscription agreements with accredited investors (the “Investors”), pursuant to which Berry sold an aggregate of 6,704,938 Units at a purchase price of $0.80 per Unit, for aggregate gross proceeds of $5,363,950 (the “Private Offering”). Each Unit consists of one share of common stock and one five-year warrant (the “Investor Warrants”) to purchase one share of common stock at an exercise price of $0.80. The exercise price of the Investor Warrants is subject to adjustment and are redeemable under certain circumstances.
Charles Vista, LLC (the “Placement Agent”) was retained as the placement agent for the Private Offering. The Placement Agent was paid a cash fee of $536,395 (equal to 10% of the gross proceeds), a non-accountable expense allowance of $160,918 (equal to 3% of the gross proceeds), and a consulting fee of $60,000. In addition, the Company issued to the Placement Agent five-year warrants (the “Placement Agent Warrants) to purchase 2,681,975 shares of common stock (equal to 20% of the shares of common stock (i) included as part of the Units sold in the Private Offering and (ii) issuable upon exercise of the Investor Warrants) at an exercise price of $0.80, exercisable on a cash or cashless basis. The Company has agreed to engage the Placement Agent as its warrant solicitation agent in the event the Investor Warrants are called for redemption and will pay a warrant solicitation fee to the Placement Agent equal to 5% of the amount of funds solicited by the Placement Agent upon the exercise of the Investor Warrants following such redemption.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Financial Statements
For the period from April 6, 2010 (inception) to December 31, 2010 and the year ended December 31, 2011
(in US dollars unless otherwise noted)
January 30, 2013
Report of Independent Registered Public Accounting Firm
To the Directors of
DelMar Pharmaceuticals (BC) Ltd.
We have audited the accompanying balance sheets, statements of operations and comprehensive loss, changes in stockholder’s deficiency and cash flows of DelMar Pharmaceuticals (BC) Ltd. (the Company) (a development stage enterprise) at December 31, 2011 and 2010 and the results of its operations and cash flows for the period from April 6, 2010 (date of incorporation) to December 31, 2010 and for the year ended December 31, 2011 and, cumulatively for the period from April 6, 2010 to December 31, 2011. Management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of DelMar Pharmaceuticals (BC) Ltd. as of December 31, 2011 and December 31, 2010 and the results of its operations and cash flows for the period from April 6, 2010 (date of incorporation) to December 31, 2010 and for the year ended December 31, 2011 and, cumulatively for the period from April 6, 2010 to December 31, 2011, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 1, the Company has a history of operating losses, has limited cash resources, and its viability is dependent upon its ability to meet its future financing requirements. These factors raise substantial doubt about the Company's ability to continue as a going concern. Management's plans regarding those matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
(signed) PricewaterhouseCoopers LLP
Chartered Accountants
PricewaterhouseCoopers LLP
PricewaterhouseCoopers Place, 250 Howe Street, Suite 700, Vancouver, British Columbia, Canada V6C 3S7
T: +1 604 806 7000, F: +1 604 806 7806
“PwC” refers to PricewaterhouseCoopers LLP, an Ontario limited liability partnership.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Balance Sheets
As at December 31, 2011 and 2010
(in US dollars unless otherwise noted)
| |
|
Note
|
|
|
|
2011 |
|
|
|
2010 |
|
| |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
|
|
|
|
15,018 |
|
|
|
24,375 |
|
|
Taxes and other receivables
|
|
|
4 |
|
|
|
38,802 |
|
|
|
14,785 |
|
|
Prepaid expenses
|
|
|
|
|
|
|
14,197 |
|
|
|
10,099 |
|
|
Prototype drug product
|
|
|
3 |
|
|
|
- |
|
|
|
250,000 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
68,017 |
|
|
|
299,259 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities
|
|
|
5 |
|
|
|
561,145 |
|
|
|
279,412 |
|
|
Loan payable
|
|
|
3 |
|
|
|
256,831 |
|
|
|
- |
|
|
Related party payables
|
|
|
8 |
|
|
|
21,028 |
|
|
|
21,363 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
839,004 |
|
|
|
300,775 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative liability
|
|
|
6 |
|
|
|
106,146 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
945,150 |
|
|
|
300,775 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Deficiency
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Authorized - unlimited number with no par value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issued and outstanding - 9,059,375 (2010 - 8,256,250)
|
|
|
7 |
|
|
|
418,611 |
|
|
|
134,161 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
7 |
|
|
|
103,727 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Subscriptions receivable
|
|
|
7 |
|
|
|
- |
|
|
|
(28,506 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Deficit accumulated during the development stage
|
|
|
|
|
|
|
(1,441,770 |
) |
|
|
(108,759 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income
|
|
|
|
|
|
|
42,299 |
|
|
|
1,588 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
(877,133 |
) |
|
|
(1,516 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
68,017 |
|
|
|
299,259 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Nature of operations and going concern (note 1)
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (note 10)
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Subsequent events (note 12)
|
|
|
|
|
|
|
|
|
|
|
|
|
Approved by the Board of Directors
| (signed) Jeffrey Bacha |
President and CEO |
(signed) Dennis Brown |
Director |
| |
|
|
|
The accompanying notes are an integral part of these financial statements.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Statements of Operations and Comprehensive Loss
(in US dollars unless otherwise noted)
| |
|
Note
|
|
|
Year ended
December 31,
2011
$
|
|
|
Period from
April 6, 2010
to
December 31,
2010
$
|
|
|
Period from April 6, 2010 (inception) to
December 31,
2011
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
|
|
|
1,051,139 |
|
|
|
41,657 |
|
|
|
1,092,796 |
|
|
General and administrative
|
|
|
|
|
|
241,802 |
|
|
|
67,599 |
|
|
|
309,401 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
(1,292,941 |
) |
|
|
(109,256 |
) |
|
|
(1,402,197 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (loss)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange (loss) gain
|
|
|
|
|
|
(18,137 |
) |
|
|
497 |
|
|
|
(17,640 |
) |
|
Interest expense
|
|
|
3, 5 |
|
|
|
(21,933 |
) |
|
|
- |
|
|
|
(21,933 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
(40,070 |
) |
|
|
497 |
|
|
|
(39,573 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the period
|
|
|
|
|
|
|
(1,333,011 |
) |
|
|
(108,759 |
) |
|
|
(1,441,770 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share
|
|
|
|
|
|
|
(0.16 |
) |
|
|
(0.02 |
) |
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares
|
|
|
|
|
|
|
8,527,466 |
|
|
|
6,145,688 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
|
|
|
|
(1,333,011 |
) |
|
|
(108,759 |
) |
|
|
(1,441,770 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive (loss) income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Translation to US dollar presentation currency
|
|
|
|
|
|
|
40,711 |
|
|
|
1,588 |
|
|
|
42,299 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss
|
|
|
|
|
|
|
(1,292,300 |
) |
|
|
(107,171 |
) |
|
|
(1,399,471 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these financial statements.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Statements of Changes in Stockholders' Deficiency
For the period from April 6, 2010 (inception) to December 31, 2010 and year ended December 31, 2011
(in US dollars unless otherwise noted)
| |
|
Number of
shares
|
|
|
Common
stock
$
|
|
|
Additional
paid-in
capital
$
|
|
|
Accumulate
other
comprehensive
income
$
|
|
|
Subscriptions
receivable
$
|
|
|
Deficit
Accumulated
during the
development
stage
$
|
|
|
Stockholders'
deficiency
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at April 6, 2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(inception)
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of founders’ shares (note 7)
|
|
|
7,000,000 |
|
|
|
6,667 |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
6,667 |
|
|
Issuance of common shares (note 7)
|
|
|
1,000,000 |
|
|
|
95,403 |
|
|
|
|
|
|
- |
|
|
|
(28,506 |
) |
|
|
- |
|
|
|
66,897 |
|
|
Shares issued from Del Mar Employee Share Purchase Trust for services - net (note 7)
|
|
|
256,250 |
|
|
|
32,091 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
32,091 |
|
|
Comprehensive loss for the period
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,588 |
|
|
|
- |
|
|
|
- |
|
|
|
1,588 |
|
|
Loss for the period
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(108,759 |
) |
|
|
(108,759 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance - December 31, 2010
|
|
|
8,256,250 |
|
|
|
134,161 |
|
|
|
- |
|
|
|
1,588 |
|
|
|
(28,506 |
) |
|
|
(108,759 |
) |
|
|
(1,516 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Collection of subscriptions receivable
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
- |
|
|
|
28,506 |
|
|
|
- |
|
|
|
28,506 |
|
|
Issuance of units net of cash issue costs (note 7)
|
|
|
400,000 |
|
|
|
119,896 |
|
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
119,896 |
|
|
Issuance of units for services (note 7)
|
|
|
200,000 |
|
|
|
60,301 |
|
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
60,301 |
|
|
Issuance of units for settlement of accounts payable (note 7)
|
|
|
50,000 |
|
|
|
15,075 |
|
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
15,075 |
|
|
Issuance of warrants related to share issuance costs of units (note 7)
|
|
|
- |
|
|
|
(5,962 |
) |
|
|
14,295 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
8,333 |
|
|
Issuance of warrants for patents (note 3)
|
|
|
- |
|
|
|
- |
|
|
|
89,432 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
89,432 |
|
|
Shares issued from Del Mar Employee Share Purchase Trust for services - net (note 7)
|
|
|
153,125 |
|
|
|
95,140 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
95,140 |
|
|
Comprehensive loss for the year
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
40,711 |
|
|
|
- |
|
|
|
- |
|
|
|
40,711 |
|
|
Loss for the year
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(1,333,011 |
) |
|
|
(1,333,011 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance - December 31, 2011
|
|
|
9,059,375 |
|
|
|
418,611 |
|
|
|
103,727 |
|
|
|
42,299 |
|
|
|
- |
|
|
|
(1,441,770 |
) |
|
|
(877,133 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these financial statements.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Statement of Cash Flows
(in US dollars unless otherwise noted)
| |
|
Year ended
December 31,
2011
$
|
|
|
Period from
April 6, 2010
to
December 31,
2010
$
|
|
|
Period from April 6, 2010 (inception) to
December 31,
2011
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
|
Loss for the year
|
|
|
(1,333,011 |
) |
|
|
(108,759 |
) |
|
|
(1,441,770 |
) |
|
Items not affecting cash
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest
|
|
|
6,831 |
|
|
|
- |
|
|
|
6,831 |
|
|
Units issued for services
|
|
|
95,140 |
|
|
|
- |
|
|
|
95,140 |
|
|
Warrants issued for patents
|
|
|
89,432 |
|
|
|
- |
|
|
|
89,432 |
|
|
Prototype drug product
|
|
|
250,000 |
|
|
|
- |
|
|
|
- |
|
|
Share-based compensation
|
|
|
95,140 |
|
|
|
32,091 |
|
|
|
127,231 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
(796,468 |
) |
|
|
(76,668 |
) |
|
|
(1,123,136 |
) |
|
Changes in non-cash working capital
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other receivables
|
|
|
(24,017 |
) |
|
|
(14,785 |
) |
|
|
(38,802 |
) |
|
Prepaid expenses
|
|
|
(4,098 |
) |
|
|
(10,099 |
) |
|
|
(14,197 |
) |
|
Accounts payable and accrued liabilities
|
|
|
596,229 |
|
|
|
31,000 |
|
|
|
877,229 |
|
|
Related party payables
|
|
|
(335 |
) |
|
|
21,363 |
|
|
|
21,028 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
(228,689 |
) |
|
|
(49,189 |
) |
|
|
(277,878 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net proceeds from the issuance of common shares
|
|
|
28,506 |
|
|
|
73,564 |
|
|
|
102,070 |
|
|
Net proceeds from the issuance of units
|
|
|
190,826 |
|
|
|
- |
|
|
|
190,826 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
219,332 |
|
|
|
73,564 |
|
|
|
292,896 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Decrease) increase in cash and cash equivalents
|
|
|
(9,357 |
) |
|
|
24,375 |
|
|
|
15,018 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents - beginning of period
|
|
|
24,375 |
|
|
|
- |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents - end of period
|
|
|
15,018 |
|
|
|
24,375 |
|
|
|
15,018 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplementary information
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of units for the settlement of accounts payable (notes 7 and 8)
|
|
|
23,785 |
|
|
|
|
|
|
|
23,785 |
|
|
Non-cash share issuance costs (note 7)
|
|
|
14,295 |
|
|
|
- |
|
|
|
14,295 |
|
|
Acquisition of common shares by Del Mar Employee Share Purchase Trust (note 7)
|
|
|
- |
|
|
|
1,904 |
|
|
|
1,904 |
|
|
Non-cash acquisition of the Prototype drug product (note 3)
|
|
|
- |
|
|
|
250,000 |
|
|
|
- |
|
|
Settlement of accounts payable with loan payable (note 3)
|
|
|
250,000 |
|
|
|
- |
|
|
|
250,000 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these financial statements.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
|
1
|
Going concern and nature of operations
|
Going concern
For the year ended December 31, 2011, the Company reported a loss of $1,333,011 and an accumulated deficit of $1,441,770 at that date. As at December 31, 2011, DelMar has cash and cash equivalents on hand of $15,018 and a negative working capital balance of $770,987. DelMar does not have the prospect of achieving revenues in the near future and DelMar will require additional funding to maintain its research and development projects and for general operations. These circumstances lend substantial doubt as to the ability of DelMar to meet its obligations as they come due (note 11).
Consequently, management is pursuing various financing alternatives to fund DelMar’s operations so it can continue as a going concern (notes 12(h) and 12(i)). In addition, the Company has not begun to commercialize or generate revenues from any product candidate. Accordingly, the Company is considered to be in the development stage as defined in Accounting Standards Codification (ASC) 915-10. Management plans to secure the necessary financing through the issue of new equity and/or the entering into of strategic partnership arrangements. Nevertheless, there is no assurance that these initiatives will be successful.
These financial statements have been prepared on a going concern basis which assumes that DelMar will continue its operations for the foreseeable future and contemplates the realization of assets and the settlement of liabilities in the normal course of business.
The conditions and risks noted above cast substantial doubt on the validity of that assumption. These financial statements do not give effect to any adjustments to the amounts and classification of assets and liabilities that may be necessary and could potentially be material, should DelMar be unable to continue as a going concern.
Nature of operations
DelMar Pharmaceuticals Ltd. (“DelMar” or the “Company”) is a development stage company focused on the discovery and development of new medicines with the potential to treat cancer patients who have failed modern targeted or biologic therapy. DelMar has initiated a clinical trial with its lead drug candidate VAL-083 for the treatment of refractory glioblastoma multiforme (GBM). The Phase I/II study is an open-label, single arm dose-escalation study designed to evaluate the safety, tolerability, pharmacokinetics and anti-tumor activity of VAL-083 in patients with histologically confirmed initial diagnosis of primary WHO Grade IV malignant glioma (GBM), now recurrent. Patients with prior low-grade glioma or anaplastic glioma are eligible, if histologic assessment demonstrates transformation to GBM.
The Company efforts have been devoted to research and development, raising capital, recruitment of personnel and long-term planning. The Company is currently a private company. The Company was incorporated on April 6, 2010 under the British Columbia Business Corporations Act and is domiciled in British Columbia, Canada. The address of its registered office is Suite 720 - 999 West Broadway, Vancouver, British Columbia, V5Z 1K5.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
On May 27, 2010 the company issued shares to the founders of DelMar. As of this date, the Company did not have any operations or assets. Accordingly these founders’ shares were issued at nominal value.
In the summer of 2010 the company began discussions with Valent Technologies LLC (“Valent”) regarding the acquisition of certain intellectual property and a prototype drug product, VAL-083. During this time the company also began to develop a business plan for the development of VAL-083 as a potential new cancer therapy.
On September 12, 2010 DelMar executed a Patent Assignment Agreement with Valent to acquire the prototype drug product and certain intellectual property.
On October 20, 2010 DelMar filed an Investigational New Drug Application (“IND”) with the United States Food & Drug Administration (“FDA”) to initiate clinical trials with VAL-083 as a potential cancer treatment.
During the remainder of 2010 and the first half of 2011, DelMar conducted research requested by the FDA focused on developing new analytical methods related to manufacturing and conducting pre-clinical toxicology studies to enable the allowance of the IND. New patent applications were filed by DelMar to protect this new intellectual property.
In September 2011 DelMar announced that its IND application had been allowed by the FDA and in October, 2011 DelMar commenced its clinical trials in the United States with its lead drug candidate, VAL-083. Also in the last quarter of 2011 DelMar initiated its preclinical research into the molecular mechanism of action of VAL-083.
The prototype drug product acquired from Valent was used in DelMar’s clinical trials undertaken in 2011 and preclinical studies conducted in 2011 and 2012.
In February 2012 DelMar received approval from the FDA Office of Orphan Products Development granting orphan drug designation for VAL-083 for the treatment of glioma, including glioblastoma multiforme (“GBM”), the most common and aggressive form of brain cancer.
In April 2012 DelMar presented results of research conducted in collaboration with the University of British Columbia. These data gathered differentiated the mechanism of action of VAL-083 from other drugs approved to treat GBM.
In the second quarter of 2012 patents from Valent were assigned to DelMar and DelMar continued to file new patents for various matters linked to Val-083.
In October 2012 DelMar announced a strategic collaboration with Guangxi Wuzhou Pharmaceutical Company, a subsidiary of publicly traded Guangxi Wuzhou Zhongheng Group Co., Ltd for the development of VAL-083, known as “DAG for Injection” in China.
In November 2012, DelMar presented interim clinical data demonstrating activity against GBM.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
In January 2013 DelMar announced that the European Committee for Orphan Medicinal Products (COMP) has recommended the designation of VAL-083 as an orphan medicinal product for the treatment of glioma.
|
2
|
Significant accounting policies
|
Basis of presentation
The financial statements of DelMar have been prepared in accordance with United States Generally Accepted Accounting Principles (“US GAAP”) and are presented in United States dollars. The Company’s functional currency is the Canadian dollar.
The principal accounting policies applied in the preparation of these financial statements are set out below and have been consistently applied to all periods presented.
Use of estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions about future events that affect the reported amounts of assets, liabilities, expenses, contingent assets and contingent liabilities as at the end or during the reporting period. Actual results could significantly differ from those estimates. Significant areas requiring management to make estimates include the derivative liability and the valuation of equity instruments issued for services. Further details of the nature of these assumptions and conditions may be found in the relevant notes to the financial statements.
|
a)
|
Fair value of derivative liability
|
The derivative is not traded in an active market and the fair value is determined using valuation techniques. The Company uses judgment to select a variety of methods to make assumptions that are based on specific management plans and market conditions at the end of each reporting period. The Company uses a fair value estimate to determine the fair value of the derivative liability. The carrying value of the derivative liability would be higher or lower as management estimates around specific probabilities change. The estimates may be significantly different from those recorded in the financial statements because of the use of judgment and the inherent uncertainty in estimating the fair value of these instruments that are not quoted in an active market. All changes in the fair value are recorded in the statement of loss each reporting period. This is considered to be a Level 3 financial instrument.
Cash and cash equivalents
Cash and cash equivalents consist of cash on deposit and highly liquid short-term interest-bearing securities with maturities at the date of purchase of three months or less. Cash and cash equivalents are held at a single recognized Canadian financial institution. Interest earned is recognized in the statements of loss.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
Foreign currency translation
The functional currency of the Company is the Canadian dollar. Transactions that are denominated in a foreign currency are re-measured into the functional currency at the current exchange rate on the date of the transaction. Any foreign currency denominated monetary assets and liabilities are subsequently re-measured at current exchange rates, with gains or losses recognized as foreign exchange losses or gains in the statement of operations. Nonmonetary assets and liabilities are translated at historical exchange rates. Expenses are translated at average exchange rates during the period. Exchange gains and losses are included in statement of operations for the period.
Adjustments arising from the translation of the Company’s financial statements to United States dollars for presentation purposes due to differences between average rates and balance sheet rates are recorded in other comprehensive income.
The financial statements have been presented in a currency other than the functional currency of the Company as management has determined that the U.S. dollar is the common currency in which the Company’s peers, being international drug and pharmaceutical companies, present their financial statements. For presentation purposes the assets and liabilities of the Company are translated to U.S. dollars at exchange rates at the reporting date. The historical equity transactions have been translated using historical rates in effect on the date that each transaction occurred. The income and expenses are translated to U.S. dollars at the average exchange rate for the period in which the transaction arose. Exchange differences arising are recognized in a separate component of equity titled accumulated other comprehensive income (loss).
Current and deferred income taxes
The Company follows the liability method of accounting for income taxes. Under this method, current income taxes are recognized for the estimated income taxes payable for the current period. Income taxes are accounted for using the asset and liability method of accounting. Future income taxes are recognized for the future income tax consequences attributable to differences between the carrying values of assets and liabilities and their respective income tax bases and for loss carry-forwards. Future income tax assets and liabilities are measured using enacted income tax rates expected to apply to taxable income in the periods in which temporary differences are expected to be recovered or settled. The effect on future income tax assets and liabilities of a change in tax laws or rates is included in earnings in the period that includes the enactment date. When realization of future income tax assets does not meet the more-likely-than-not criterion for recognition, a valuation allowance is provided.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
Financial instruments
The Company has financial instruments that are measured at fair value. To determine the fair value, we use the fair value hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use to value an asset or liability and are developed based on market data obtained from independent sources. Unobservable inputs are inputs based on assumptions about the factors market participants would use to value an asset or liability. The three levels of inputs that may be used to measure fair value are as follows:
|
·
|
Level one - inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities;
|
|
·
|
Level two - inputs are inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly such as interest rates, foreign exchange rates, and yield curves that are observable at commonly quoted intervals; and
|
|
·
|
Level three - unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use.
|
Assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. Changes in the observability of valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy.
The Company’s financial instruments consist of cash and cash equivalents, accounts receivable, accounts payable and derivative liability. The carrying values of cash and cash equivalents, accounts receivable, and accounts payable approximate their fair values due to the immediate or short-term maturity of these financial instruments.
As quoted prices for the derivative liability are not readily available, the Company used a probability adjusted Black-Scholes pricing model, as described in note 6 to estimate fair value. The derivative liability utilizes Level 3 inputs as defined above.
The Company has the following liabilities under the fair value hierarchy:
|
Asset/liability
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
| |
|
|
|
|
|
|
|
|
|
|
Derivative liability
|
|
|
- |
|
|
|
- |
|
|
|
106,146 |
|
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
Prototype drug product
The prototype drug product (the “drug”) is stated at the lower of cost and net realizable value. The cost of the drug is comprised of direct costs related to the acquisition of the drug. During the year the Company recorded $nil in relation to these amounts as inventories (2010 - $250,000 was recorded as prototype drug product) and fully utilized in clinical and pre-clinical testing trials during the year ended December 31, 2011.
Intangible assets
Under its assignment agreement with Valent Technologies LLC (“Valent”) (note 3) the Company has incurred certain costs relating to patents that will be assigned to the Company under its agreement with Valent. As the patents have not yet been assigned to the Company, the Company has expensed these costs for the year ended December 31, 2011.
Expenditures associated with the maintenance of licensing or technology agreements are expensed as incurred. Costs previously recognized as an expense are not recognized as an asset in subsequent periods.
Research and development costs
Research and development costs are expensed in the period incurred.
Clinical trial expenses
Clinical trial expenses are a component of research and development costs and include fees paid to contract research organizations, investigators and other vendors who conduct specific research for product development activities on behalf of the Company. The amount of clinical trial expenses recognized in a period related to service agreements is based on estimates of the work performed on an accrual basis. These estimates are based on patient enrollment, services provided and goods delivered, contractual terms and experience with similar contracts. The Company monitors these factors to the extent possible and adjusts our estimates accordingly. Prepaid expenses or accrued liabilities are adjusted if payments to service providers differ from estimates of the amount of service completed in a given period.
Government assistance and investment tax credits
The Company uses the cost reduction method of accounting for tax credits. Tax credits related to the acquisition of research equipment are deducted from the related asset with amortization being calculated on the net amount or to the expenditures in the determination of net income as the expenditures are incurred. These amounts are recognized when there is reasonable assurance they will be realized.
Non-refundable government grants are recorded as a reduction of expenses or in the cost of the asset. Grants in excess of expenditures are deferred to future periods, to be offset against any future expenditure to be incurred or credited to development costs if they exceed future expenditures.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
The benefits of refundable investment tax credits for scientific research and experimental development expenditures are recognized in the year the qualifying expenditure is made when there is reasonable assurance the investment tax credits will be realized. The investment tax credits recorded are based on management’s estimates of amounts expected to be recovered and are subject to audit by taxation authorities. The investment tax credit reduces the carrying cost of expenditures for equipment or research and development expenses to which it relates.
Shares for services
The Company has issued equity instruments for services provided by employees and nonemployees. The equity instruments are valued at the fair value of the instrument granted (see notes 6 and 7 for assumptions).
The Company has transferred shares from the DelMar Employee Share Purchase Trust (the “Trust”) (note 7) to consultants and management in exchange for services rendered to the Company. The Company recognizes the fair value of the shares transferred as an expense with a corresponding increase in common stock. The shares reserved for issuance to consultants and management that are held by the Trust are included in the financial statements at year end. There are no other assets in the Trust. The number of shares outstanding for issue from the Trust at December 31, 2011 is 1,590,625 (2010 - 1,743,750) (note 7).
The shares transferred from the Trust have been valued using the fair value of the shares transferred. The Company used recent share transactions in order to determine the fair value of the shares transferred from the Trust.
Comprehensive income
In accordance with ASC 220, “Comprehensive Income” (“ASC 220”) all components of comprehensive income, including net loss, are reported in the financial statements in the period in which they are recognized. Comprehensive income is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Net loss and other comprehensive income, including foreign currency translation adjustments, are reported, net of any related tax effect, to arrive at comprehensive income. No taxes were recorded on items of other comprehensive income.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
Derivative liability
The Company accounts for certain warrants under the authoritative guidance on accounting for derivative financial instruments indexed to, and potentially settled in, a company’s own stock, on the understanding that in compliance with applicable securities laws, the warrants require the issuance of securities upon exercise and do not sufficiently preclude an implied right to net cash settlement. The Company classifies warrants in its balance sheet as a derivative liability which is fair valued at each reporting period subsequent to the initial issuance. The Company uses a probability-weighted Black-Scholes pricing model to value the warrants. Determining the appropriate fair-value model and calculating the fair value of warrants requires considerable judgment. Any change in the estimates (specifically probabilities) used may cause the value to be higher or lower than that reported. The estimated volatility of the Company’s common stock at the date of issuance, and at each subsequent reporting period, is based on the historical volatility of similar life sciences companies. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the warrants at the valuation date. The expected life of the warrants is assumed to be equivalent to their remaining contractual term.
Loss per share
Income or loss per share is calculated based on the weighted average number of common shares outstanding. Diluted loss per share does not differ from basic loss per share since the effect of the Company’s warrants is anti-dilutive. Diluted income per share is calculated using the treasury stock method which uses the weighted average number of common shares outstanding during the period and also includes the dilutive effect of potentially issuable common shares from outstanding stock options and warrants. At December 31, 2011, potential common shares of 650,000 (2010 - nil) related to outstanding warrants were excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive.
Segment information
The Company identifies its operating segments based on business activities, management responsibility and geographical location. The Company operates within a single operating segment being the research and development of cancer indications, and operates in one geographic area, being Canada. All of the Company’s assets are located in Canada.
Recent accounting pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies that are adopted by the Company as of the specified effective date. Unless otherwise discussed, we believe that the impact of recently issued standards that are not yet effective will not have a material impact on our financial position or results of operations upon adoption.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
In December 2011, FASB issued Accounting Standards Update (“ASU”) 2011-11 which amends the guidance in ASC 210, Balance Sheet (ASC 210). The ASU requires an entity to disclose information about offsetting and related arrangements to enable users of its financial statements to understand the effect of those arrangements on its financial position. The ASU is effective for annual periods beginning on or after January 1, 2013. An entity should provide the disclosures required by those amendments retrospectively for all comparative periods presented.
In June 2011, the FASB issued Accounting Standards ASU 2011-05 to amend the guidance on the presentation of comprehensive income in ASC 220. ASU 2011-05 requires companies to present a single statement of comprehensive income or two separate but consecutive statements, a statement of operations and a statement of comprehensive income. ASU 2011-05 eliminates the alternative to present comprehensive income within the statement of equity. ASU 2011-05 does not change the items that must be reported in other comprehensive income or when an item of other comprehensive income must be reclassified to net income. The ASU should be applied retrospectively and is effective for annual periods beginning after December 15, 2011. In December 2011, the FASB issued ASU 2011-12, which deferred the changes in ASU 2011-05 that relate to the presentation of reclassifications out of accumulated other comprehensive income.
In May 2011, the FASB issued ASU 2011-04, which amends the guidance on fair value measurement in ASC 820 to converge the fair value measurement and disclosure requirements under GAAP and International Financial Reporting Standards (“IFRS”) fair value measurement and disclosure requirements. The amendments change the wording used to describe the requirements for measuring fair value, changes certain fair value measurement principles and enhances disclosure requirements. This guidance is effective for annual periods beginning after December 15, 2011, applied prospectively.
|
3
|
Valent Technologies LLC agreement
|
On September 12, 2010 the Company entered into a Patent Assignment Agreement (the “Assignment”) with Valent Technologies LLC (“Valent”) to acquire patents and the prototype drug product related to VAL-083. In accordance with the Assignment the consideration was $250,000 to acquire the prototype drug product. In addition, under certain circumstances Valent will assign, convey and transfer to the Company all its right, title and interest in and to the patents in exchange for share purchase warrants. The Company will then be responsible for the further development and commercialization of VAL-083. Valent retains an option to reacquire certain intellectual property until a Financing Transaction is completed by the Company. Under the Assignment, a ‘Financing Transaction’ is defined as a cumulative equity or debt financing(s), or a merger, acquisition, amalgamation, reverse takeover or other combination, or any combination of the foregoing, cumulatively totaling at least $2,000,000. In accordance with the terms of the Assignment, Valent is entitled to receive a future royalty on revenues derived from the development and commercialization of VAL-083. In the event that the Company terminates the agreement, the Company may be entitled to receive royalties from Valent’s subsequent development of VAL-083 depending on the development milestones the Company has achieved prior to the termination of the Assignment.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
Pursuant to a loan agreement dated February 3, 2011, the Company has entered a loan with Valent for the $250,000 for the purchase of the prototype drug product. The loan is unsecured and bears interest at 3.00% per year. As a result the balance of the loan payable at December 31, 2011 is $256,831, including accrued interest of $6,831.
In addition, under the terms of the Assignment, the Company will issue 500,000 warrants to acquire common stock of the Company to Valent following the completion of a Financing Transaction. The Company is not required to issue any warrants unless a Financing Transaction is completed. If issued, each warrant will be exercisable into one common share of the Company. The price of the warrants will be based on the price of the Financing Transaction. The warrants will have a term of five years commencing on the date of the completion of the Financing Transaction and will be exercisable for their entire term. If no Financing Transaction is completed and Valent exercises its option to reacquire VAL-083 then the warrants shall expire automatically. Under certain conditions, the Company has the right to force the exercise of the Valent warrants.
The financing completed by the Company that closed in February 2012 (note 12(a)) qualified as a Financing Transaction under the Assignment and a result, the Company issued 500,000 warrants to Valent on February 1, 2012 at an exercise price of CDN $0.50 per warrant. In exchange for the warrants, Valent has begun the process of assigning all of its right, title and interest in and to the patents for VAL-083. The fair value of the contingent warrants of $89,432 has been recognized as an expense and a corresponding increase to additional paid in capital at December 31, 2011.
|
4
|
Taxes and other receivables
|
| |
|
|
2011 |
|
|
|
2010 |
|
| |
|
|
$ |
|
|
|
$ |
|
| |
|
|
|
|
|
|
|
|
|
Government grants
|
|
|
26,900 |
|
|
|
8,617 |
|
|
Other receivables
|
|
|
11,902 |
|
|
|
6,168 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
38,802 |
|
|
|
14,785 |
|
On September 1, 2010 the Company was granted a non-repayable financial contribution of up to $44,249 (CDN $45,000) from the National Research Council of Canada Industrial Research Assistance Program (“IRAP”). Awards under the IRAP grant directly reduce the Company’s research and development costs by 75% of eligible expenses. Total expenses under this program will be $58,998 (CDN $60,000) of which $44,249 (CDN $45,000) will be reimbursed through IRAP. The Company will be reimbursed for certain research and development costs to a maximum of $29,499 (CDN $30,000) in the period from September 1, 2010 thru March 31, 2011 and a further $14,750 (CDN $15,000) in the period from April 1, 2011 thru July 31, 2011. Under this IRAP grant the Company requested an aggregate total reimbursement of $44,249 ($27,392 in 2011 and $16,857 in 2010) and has received $44,249 (2010 - $8,240) to December 31, 2011 resulting in a receivable of $nil (2010 - $8,617) at December 31, 2011.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
On July 15, 2011 the Company was granted a second non-repayable financial contribution of up to $39,332 (CDN $40,000) from IRAP. Total expenses under this program will be $52,442 (CDN $53,333) of which $39,332 (CDN $40,000) will be reimbursed. The Company will be reimbursed for certain research and development costs to a maximum of $39,332 (CDN $40,000) in the period from July 15, 2011 thru December 15, 2011. To December 31, 2011 the Company has requested reimbursement of $39,332 (CDN $40,000) under the second IRAP grant and has received $12,432 resulting in a receivable of $26,900 at December 31, 2011.
Total amounts credited in the statement operations for all IRAP grants in 2011 was $66,724 (2010 - $16,857).
|
5
|
Accounts payable and accrued liabilities
|
| |
|
|
2011 |
|
|
|
2010 |
|
| |
|
|
$ |
|
|
|
$ |
|
| |
|
|
|
|
|
|
|
|
|
Trade payables
|
|
|
561,145 |
|
|
|
279,412 |
|
|
Payable to related parties (note 8)
|
|
|
21,028 |
|
|
|
21,363 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
582,173 |
|
|
|
300,775 |
|
During the year ended December 31, 2011 the Company incurred $15,102 in interest expense relating to outstanding trade payable balances. Subsequent to the year end the Company issued 500,000 common shares valued at $253,050 (CDN $250,000) and represents partial settlement of the Company’s accounts payable balance with Valent (note 12(f)). The value of the shares issued as partial settlement was based on the most recent financing which was in progress at December 31, 2011.
The Company issued 500,000 units on October 3, 2011, 100,000 on October 7, 2011, and 50,000 on November 11, 2011. The total 650,000 units were issued for CDN $0.50 per unit or total consideration of $310,570 (CDN $325,000). Each unit consists of one common share and share purchase warrant. Of the total consideration of $310,570, $118,925 relates to non-cash consideration received for the provision of services by officers and directors of the Company and the settlement of accounts payable with an officer of the Company (note 8).
Each warrant is exercisable until October 31, 2013. The exercise price of each warrant is as follows:
|
Exercise dates
|
|
Price
$Cdn
|
|
| |
|
|
|
|
Up to and including October 31, 2012
|
|
0.75
|
|
|
From November 1, 2012 up to December 31, 2012
|
|
0.80
|
|
|
From January 1, 2013 up to April 29, 2013
|
|
0.90
|
|
|
From April 30, 2013 up to July 30, 2013
|
|
1.00
|
|
|
From July 31, 2013 up to October 31, 2013
|
|
1.25
|
|
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
Under the terms of the subscription agreements DelMar shall use reasonable commercial efforts to complete a liquidity event (“Liquidity Event”). A Liquidity Event shall include but not be limited to the sale of the Company, or its assets, or listing of the Company’s shares on a public stock exchange, through an initial public offering (”IPO”), reverse takeover, merger, amalgamation, or any other comparable event that includes a minimum additional aggregate funding of not less than CDN $5,000,000.
If the Company has not filed a preliminary prospectus with respect to an Initial Public Offering (“IPO”) with one or more securities regulators in Canada or the United States or entered into a letter of intent or binding agreement with respect to a Liquidity Event by certain dates then a portion of the warrants associated with the units will have a cashless exercise provision that will be automatically activated. The cashless exercise provisions are as follows:
|
Liquidity event date
|
|
Portion of warrants
to be exercised
without cash
|
|
| |
|
|
|
|
By October 31, 2012
|
|
|
10 |
% |
|
By January 1, 2013
|
|
an additional 15
|
% |
|
By April 30, 2013
|
|
an additional 20
|
% |
|
By July 31, 2013
|
|
an additional 25
|
% |
|
By October 31, 2013
|
|
an additional 30
|
% |
If the Company has not met the requirements for a Liquidity Event by October 31, 2013, all of the warrants issued with the units will be automatically exercised for one common share for no additional consideration.
Upon the receipt of the Liquidity Event notice, each warrant holder will have 20 days after receipt thereof to conditionally exercise any outstanding warrants subject to the occurrence of the Liquidity Event. To the extent that a warrant holder elects not to exercise his rights to conditionally exercise all or any warrants during the Liquidity Event notice period the warrant holder’s right to exercise such warrants will be suspended until the completion of the Liquidity Event or the Company notifies the warrant holder that it will not be proceeding with the Liquidity Event.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
Following the occurrence of a Liquidity Event any warrants that were not exercised at such time shall be adjusted as follows:
|
i)
|
Unexercised warrants shall expire on the date which is 12 months after the occurrence of a Liquidity Event (the “New Expiry Date”);
|
|
ii)
|
Up to and including the sixth month anniversary of the Liquidity Event, the exercise price shall equal 120% of the closing price of the underlying securities on the Liquidity Event date;
|
|
iii)
|
From and excluding the sixth month anniversary date of the Liquidity Event to and including the New Expiry Date, the exercise price of the warrants shall be 150% of the closing price of the underlying securities on the Liquidity Event date.
|
If at any time after the completion of a Liquidity Event the common shares of the Company, or exchange shares in the event of a reverse takeover, the closing price of the Company’s shares or exchange shares is at least two times the closing price of the common shares of the Company or the exchange shares on the completion date of a Liquidity Event, as the case may be, the Company shall be permitted to terminate any outstanding warrants on three business days written notice.
Based on the terms of the warrants it was determined that the warrants were considered a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value every reporting period with gains or losses on the changes in fair value recorded in the statement of loss and comprehensive loss.
Pursuant to finders’ fee agreements (notes 10(a) and 12(c)) the Company is required to pay finder’s fees related to the issuance of certain units. In relation to these agreements the Company has accrued $3,931 related to the cash component and $14,295 related to the contingent warrant component at December 31, 2011. These items have been recorded as issue costs and been allocated to capital stock and derivative liability.
The warrants issued with the units have been valued using a probability-weighted Black-Scholes pricing model using the following assumptions: dividend rate - 0%, volatility - 97.3%, risk free rate - 1.25% and an initial term of 2 years.
At December 31, 2011 the Company has recognized the fair value of the contingent Valent warrants (note 3) and the contingent financing finders’ fee warrants (notes 10(a) and 12(c)). The contingent warrants have been recognized in additional paid in capital at December 31, 2011 and will be reclassified to warrants when the warrants are actually issued during the year ended December 31, 2012.
Authorized
Unlimited common shares without par value
Issued and outstanding at December 31, 2011 - 9,059,375 (December 31, 2010 - 8,256,250)
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
|
a)
|
Shares issued to founders
|
On May 27, 2010 the Company issued 7,000,000 common shares to its founders at $0.001 per share for total proceeds of $6,667. Of the 7,000,000 shares issued, 6,000,000 were issued to founders who are also officers or directors of the Company. In addition, of the 7,000,000 shares issued, 6,700,000 are subject to vesting provisions and a repurchase option to the Company. At any time prior to the expiration of 36 months from May 27, 2010 the Company at its sole discretion may repurchase some or all of the unvested 6,700,000 shares at $0.001 per share.
For the 6,700,000 shares subject to vesting, 25% of the common shares shall vested immediately on May 27, 2010 and the remaining shares shall vest in twelve equal tranches on each quarterly anniversary of May 27, 2010 with the number of shares to vest on each such date to equal 1/16 of the number of shares issued on May 27, 2010. If any of the subscribers is or becomes a director, officer, employee or consultant of the Company or an affiliate of the Company, all unvested shares shall vest immediately if the subscriber is subsequently removed as a director or officer of the Company or its affiliate, or is subsequently terminated as an employee or consultant of the Company or its affiliate, in each case without cause.
|
b)
|
Shares issued to the DelMar Employees Share Purchase Trust
|
The Company has established the DelMar Employees Share Purchase Trust (the “Trust”). The purposes of the Trust are to (i) enhance the ability of the Company and its affiliates to attract, motivate, retain and reward directors, officers, employees and consultants, (b) facilitate employee ownership of shares of the company and (c) promote closer alignment of interests between key employees of the company and its shareholders. The Trust is overseen by a Trustee appointed by the Company and funds from the Company (“Settled Funds”) were used to subscribe for common shares (“Trust Shares”) in the capital of the Company. On May 27, 2010, the Company issued 2,000,000 common shares to the trust. The Company used Settled Funds to pay for the trust Shares.
| |
|
Number of shares held in Trust
|
|
| |
|
|
|
|
Balance - April 6, 2010
|
|
|
- |
|
|
Shares issued to the DelMar Employee Share Purchase Trust
|
|
|
2,000,000 |
|
|
Shares transferred to employees and consultants for services
|
|
|
(325,000 |
) |
|
Founders shares acquired by the Trust
|
|
|
68,750 |
|
| |
|
|
|
|
|
Balance - December 31, 2010
|
|
|
1,743,750 |
|
|
Shares transferred to employees and consultants for services
|
|
|
(200,000 |
) |
|
Founders shares acquired by the Trust
|
|
|
46,875 |
|
| |
|
|
|
|
|
Balance - December 31, 2011
|
|
|
1,590,625 |
|
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
The Company has transferred shares from the Trust to various consultants for work or services performed for the Company. Shares held by the Trust are not issued and outstanding until the shares are transferred out of the Trust. The Company recognized the fair value of the shares transferred as an expense with the offsetting charge to capital stock for $95,140 and $32,091 at December 31, 2011 and 2010 respectively.
|
c)
|
Shares issued in private placements
|
On August 27, 2010 the Company issued 720,000 common shares at $0.095 (CDN $0.10) per share for total proceeds of $68,414 and on September 8, 2010 the Company issued an additional 280,000 common shares at $0.096 (CDN $0.10) per share for total proceeds of $26,989. Of the total proceeds of $68,414 from the August 27, 2010 issuance, $28,506 was received in 2011 and has been recorded as subscriptions receivable at December 31, 2010.
|
8
|
Related party transactions
|
During the year ended December 31, 2011
Pursuant to consulting agreements dated August 1, 2011 with each of the Company’s officers and directors, a total of three respective agreements, Company has agreed to compensate its officers and directors for services rendered to the Company. An aggregate $26,550 (CDN $27,000) per month commencing August 1, 2011 and ending December 31, 2012 will be payable pursuant the consulting agreements. Under the consulting agreements the Company and the respective officer or director have mutually agreed that a portion of the compensation payable under the respective agreement shall be deemed to have been invested in the unit offering of the Company as of October 3, 2011. The units issued under these agreements shall have the same terms as the CDN $0.50 units issued by the Company to subscribers of the offering (note 6).
For the period from August 1 to December 31, 2011 $19,028 (CDN $20,000) per month was settled by the Company with units resulting in 200,000 units being issued. Total research and development expenses of $71,355 (CDN $75,000) and general and administrative expenses of $23,785 (CDN $25,000) have been recorded for this issuance of units.
The Company also issued 50,000 units to one of its officers for the settlement of accounts payable in the amount of $23,785 (CDN $25,000). The units were measured at fair value using the valuation estimate consistent with the most recent financing.
Included in accounts payable at December 31, 2011 is an aggregate amount owing of $21,028 to two of the Company’s directors.
Also included in accounts payable at December 31, 2011 is an amount of $496,932 relating to clinical development costs incurred by Valent on behalf of the Company. The Company also has a loan payable, including accrued interest, of $256,831 (including accrued interest) due to Valent at December 31, 2011. One of the directors and officers of the Company is also a Principal of Valent.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
During the period ended December 31, 2010
The Company acquired its prototype drug product and intellectual property rights to VAL-083 from Valent (note 3). Included in accounts payable is an amount of $250,000 relating to the acquisition of the prototype drug product.
Included in accounts payable at December 31, 2010 is an aggregate amount owing of $21,363 to two of the Company’s executive officers.
The Company has the following non-capital losses available to reduce taxable income of future years:
|
Expiry date
|
|
$ |
|
|
|
|
| |
|
|
|
|
|
|
|
2030
|
|
|
67,296 |
|
|
|
|
|
2031
|
|
|
1,098,669 |
|
|
|
|
Significant components of the Company’s deferred tax assets are shown below:
| |
|
|
2011 |
|
|
|
2010 |
|
| |
|
|
$ |
|
|
|
$ |
|
| |
|
|
|
|
|
|
|
|
|
Non-capital losses carried forward
|
|
|
148,320 |
|
|
|
9,085 |
|
|
Financing costs
|
|
|
2,306 |
|
|
|
1,128 |
|
|
Scientific research and development
|
|
|
11,182 |
|
|
|
17,921 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
161,808 |
|
|
|
28,134 |
|
|
Valuation allowance
|
|
|
(161,808 |
) |
|
|
(28,134 |
) |
| |
|
|
|
|
|
|
|
|
|
Net deferred tax assets
|
|
|
- |
|
|
|
- |
|
The income tax benefit of these tax attributes have not been recorded in these financial statements because of the uncertainty of their recovery.
The Company’s effective income tax rate differs from the statutory income tax rate of 13.5% (2010 - 13.5%).
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
The differences arise from the following items:
| |
|
|
2011 |
|
|
|
2010 |
|
| |
|
|
$ |
|
|
|
$ |
|
| |
|
|
|
|
|
|
|
|
|
Tax recovery at statutory income tax rates
|
|
|
(179,956 |
) |
|
|
(14,682 |
) |
|
Permanent differences
|
|
|
26,713 |
|
|
|
4,396 |
|
|
Other
|
|
|
19,569 |
|
|
|
(17,848 |
) |
|
Change in valuation allowance
|
|
|
133,674 |
|
|
|
28,134 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
- |
|
|
|
- |
|
The Company has applied for refundable tax credits related to its scientific research and experimental development conducted in Canada. The Company has not recognized any amounts related to its claim due to the uncertainty of collecting on the claim.
|
10
|
Commitments and contingencies
|
In June 2011 the Company entered into an agreement for assistance with financing. Under the agreement, the Company is required to pay an 8% cash commission on gross proceeds from financing arranged by the service provider. The Company will also be required to issue warrants equal to 8% of the number of units issued to investors identified by the service provider. Fees payable under this agreement are to be paid in CDN. As of December 31, 2011 the Company has accrued $3,931 (CDN $4,000) and recognized the fair value of 8,000 warrants in the amount of $14,295. The total amount of $18,226 has been recognized as share issue costs allocated between the issuance of shares and derivative liability. In February 2012 the Company paid $49,635 (CDN $50,000) under this agreement and on March 1, 2012 the Company issued 100,000 warrants. The cash paid and warrants issued represent the final amounts due under this agreement. Each warrant entitles the holder to acquire one common share at CDN $0.50 per share until March 1, 2015.
In November 2011 the Company entered into a contract for investor relations services requiring the payment of $10,000 per month commencing December 2011. The agreement will automatically renew unless 60-day written notice of termination is provided by either party. In addition, the Company is required to issue 345,000 warrants. The warrants under the agreement become issuable upon the completion of a CDN $2,500,000 financing by the Company. The warrants were issued on February 1, 2012 and they vest in 12 equal installments over a 12-month period commencing on March 1, 2012. The warrants expire on February 1, 2015.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
The Company leased an office on a month-to-month basis for the period from September 1, 2010 to November 30, 2010. In November 2010 the Company earned one year of free office rent pursuant to the submission of its business plan as part of the Discovery Parks Generator Competition. The rent-free period commenced February 1, 2011.
The Company currently rents its office space pursuant to a month to month lease at a rate of CDN$1,600 per month. During the year ended December 31, 2011, the Company recorded $480 as a rent expense (2010 - $2,190).
|
11
|
Financial risk management
|
Market risk
Market risk is the risk that changes in market prices, such as foreign exchange rates, interest rates and equity prices will affect the Company’s income or valuation of its financial instruments.
The Company is exposed to financial risk related to fluctuation of foreign exchange rates. Foreign currency risk is limited to the portion of the Company’s business transactions denominated in currencies other than the Canadian dollar, primarily expenses for research and development incurred in US dollars. The Company believes that the results of operations, financial position and cash flows would be affected by a sudden change in foreign exchange rates, but would not impair or enhance its ability to pay its US dollar accounts payable. The Company manages foreign exchange risk by converting its Canadian dollars to US dollars as needed. The Company has only recently opened a US dollar bank account. As at December 31, 2011, US dollar denominated accounts payable and accrued liabilities and loan payable exposure in US dollars totaled $753,763.
Foreign exchange risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rates. If foreign exchange rates were to fluctuate within +/-10% of the closing rate at year end the maximum exposure is $75,376.
Balances in foreign currencies at December 31, 2011 and 2010 are as follows:
| |
|
2011
US balances
$
|
|
|
2010
US balances
$
|
|
| |
|
|
|
|
|
|
|
Trade payables
|
|
|
496,932 |
|
|
|
250,000 |
|
|
Loan payable, including accrued interest
|
|
|
256,831 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
753,763 |
|
|
|
250,000 |
|
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
The Company is subject to interest rate risk on its cash and cash equivalents and believes that the results of operations, financial position and cash flows would not be significantly affected by a sudden change in market interest rates relative to the investment interest rates due to the short-term nature of the investments. As at December 31, 2011, cash and cash equivalents held in Canadian dollar savings accounts or short-term investments is $15,018. The Company’s cash balance does not currently earn interest. If interest rates were to fluctuate within +/-10% of the closing rate at year end the impact of the Company’s interest bearing accounts will be insignificant.
The only financial instruments that expose the Company to interest rate risk are its cash and cash equivalents.
Liquidity risk
Liquidity risk is the risk that the Company will encounter difficulty in raising funds to meet cash flow requirements associated with financial instruments. The recent problems in the global credit markets have resulted in a drastic reduction in the ability of companies to raise capital through the public markets. See note 1 going concern, for additional comments relating to liquidity risk. The Company continues to manage its liquidity risk based on the outflows experienced for the year ended December 31, 2011 and is undertaking efforts to conserve cash resources wherever possible. The maximum exposure of the Company’s liquidity risk is $582,173 at December 31, 2011 (2010 - $300,775).
Credit risk
Credit risk arises from cash and cash equivalents, deposits with banks and financial institutions, as well as outstanding receivables. The Company limits its exposure to credit risk, with respect to cash and cash equivalents, by placing them with high quality credit financial institutions. The Company’s cash equivalents consist primarily of operating funds with commercial banks. Of the amounts with financial institutions on deposit, the following table summarizes the amounts at risk should the financial institutions with which the deposits are held cease trading:
The maximum exposure of the Company’s credit risk is $38,802 at December 31, 2011 (2010 - $14,785).
| |
|
Cash and
cash
equivalents
$
|
|
Insured
amount
$
|
|
Non-insured
amount
$
|
| |
|
|
|
|
|
|
| |
|
15,018
|
|
15,018
|
|
-
|
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
Concentration of credit risk
Financial instruments that subject the Company to credit risk consist primarily of cash and cash equivalents.
The Company places its cash and cash equivalents in accredited financial institutions and therefore the
Company’s management believes these funds are subject to minimal credit risk. The Company has no significant off-balance sheet concentrations of credit risk such as foreign currency exchange contracts, option contracts or other hedging arrangements.
Between January 23, 2012 and May 10, 2012 the Company issued an additional 4,760,000 units at CDN $0.50 per unit for gross proceeds of $2,365,034 (CDN $2,380,000). The units consisted of one common share and one share purchase warrant. The proceeds from the issuance of 3,000,000 of these units were held in escrow and subsequently the units were cancelled by the Company and the funds returned to the subscriber. Of the remaining 1,760,000 units, 460,000 of the share purchase warrants issued with the units have the same expiry, price, and cashless exercise provisions of the warrants issued during 2011
(note 6) and the remaining 1,300,000 units have warrants that are exercisable until December 31, 2013. The exercise price of each of the 1,300,000 warrants is as follows:
|
Exercise dates
|
|
Price
$Cdn
|
|
| |
|
|
|
|
Up to and including December 31, 2012
|
|
|
0.75 |
|
|
From January 1, 2013 up to March 30, 2012
|
|
|
0.80 |
|
|
From March 31, 2013 up to June 29, 2013
|
|
|
0.90 |
|
|
From June 30, 2013 up to September 29, 2013
|
|
|
1.00 |
|
|
From September 30, 2013 up to December 31, 2013
|
|
|
1.25 |
|
The 1,300,000 warrants are subject to the same Liquidity Event provisions as the warrants previously issued (note 6). The cashless exercise provisions as follows:
|
Liquidity event date
|
|
Portion of warrants to be exercised
without cash
|
|
| |
|
|
|
|
By December 31, 2012
|
|
|
10 |
% |
|
By March 31, 2013
|
|
An additional 15
|
% |
|
By June 30, 2013
|
|
An additional 20
|
% |
|
By September 30, 2013
|
|
An additional 25
|
% |
|
By December 31, 2013
|
|
An additional 30
|
% |
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
All other significant terms of the warrants are the same as those issued during 2011. Included in the 1,760,000 warrants issued subsequent to December 31, 2011 were 360,000 units issued to officers and directors of the Company pursuant to their respective consulting agreements (notes 8).
On February 1, 2012 the Company’s board of directors approved its stock option plan (the “Plan”). Under the Plan the number of common shares that will be reserved for issuance to officers, directors, employees and consultants under the Plan will not exceed 7.5% of the share capital of the Company on a fully diluted basis. On February 1, 2012 the Company granted 930,000 options and on June 15, 2012 an additional 90,000 options were granted under the Plan. All of the stock options granted have an exercise price of CDN $0.50 and expire 10 years from the date of grant. Of the 1,020,000 stock options granted, 450,000 vest in equal monthly installments over one year and 570,000 vests in equal monthly installments over three years.
In the event of the sale of 66 2/3% of the equity securities of the Company where equity securities include shares, warrants, stock options, and any convertible securities of the Company, any options not yet granted under the Plan shall be deemed granted to the principle founders of the Company on a pro-rata basis in accordance with their ownership of the Company on a fully-diluted basis immediately prior to the closing of such a sale.
On March 1, 2012 the Company issued 5,000 warrants that entitle the holder to acquire 5,000 common shares of the Company at CDN $0.50 per share. The warrants were issued as a finder’s fee for financing assistance. The warrants expire on March 1, 2014. The Company has recorded the fair value of these contingent warrants at December 31, 2011 as the units to which the finder’s fee applies were issued during the year ended December 31, 2011. The fair value of these warrants has been recognized as share issue costs and a corresponding amount as additional paid-in capital.
DelMar Pharmaceuticals (BC) Ltd.
(a development stage company)
Notes to Financial Statements
December 31, 2011 and 2010
(in US dollars unless otherwise noted)
On May 1, 2012 the Company entered into a one-year consulting agreement with an individual for the provision of general business and strategic advisory services. Pursuant to the agreement, the Company will pay the consultant $15,000 per month and will settle the monthly fee by way of $5,000 in cash and $10,000 by way of 20,000 common shares of the Company. Until the occurrence of a Liquidity Event (defined as the completion or occurrence of an asset sale, a share sale or a Public Company Triggering Event (defined as the completion of an Initial Public Offering or Reverse Take-Over)), the common shares issued to the consultant under the agreement shall be non-transferable and shall be held in escrow by the Company in trust for the consultant. If a Liquidity Event has not occurred prior to the liquidation, dissolution or winding-up of the Company or any other distribution of the assets of the Company among its shareholders for the purpose of winding up its affairs, the common shares issued to the consultant will be forfeited to the Company for no consideration. The agreement will automatically expire on the first anniversary of the agreement unless mutually agreed to in writing by both parties. The agreement can be terminated by either party by providing 30 days written notice of termination.
|
e)
|
Related party transactions
|
Subsequent to December 31, 2011, the Company issued an additional 360,000 units to officers and directors of the Company for services rendered to the Company (notes 6 and 8). The shares issued relate to two of the Company’s directors as one of the directors has elected to receive cash for his consulting fees for the period from January 1 to December 31, 2012. Under the two consulting agreements for which units are being issued as compensation, the compensation expense is CDN $15,000 per month which is being settled with 30,000 units of the Company each month. The terms of the units issued under these agreements are the same as those units issued to the officers and directors pursuant to units previously issued to them for services (note 8). All of the shares issued under the agreements for 2012 were issued in February 2012.
|
f)
|
Settlement of accounts payable
|
On April 30, 2012, Valent was issued 500,000 common shares for partial settlement of the Company’s accounts payable balance with Valent. The total settlement amount was $253,050 (CDN $250,000).
|
g)
|
DelMar employee share purchase trust
|
Subsequent to December 31, 2011, the remaining 1,590,625 shares in the DelMar Employee Share Purchase Trust were transferred to employees and consultants of the Company. Of the 1,590,625 transferred out of the trust, 1,390,625 were transferred in equal tranches to each of the Company’s three directors. The related compensation expense will be recorded in the statement of operations.
On January 25, 2013, the Company entered into and closed an Exchange Agreement with DelMar Pharmaceuticals, Inc. (formerly Berry Only Inc. (“Berry”)) (the “Acquisition”). The Acquisition will result in Berry acquiring DelMar by issuing a sufficient number of shares such that the shareholders of DelMar will have a controlling interest in Berry subsequent to the completion of the Acquisition. Simultaneous with the Acquisition, Valent will be issued 1,150,000 common shares of Berry. The shares issued to Valent by Berry are being issued in exchange for Valent reducing certain royalties under its agreement with DelMar. Upon completion of the Acquisition DelMar will become a wholly-owned subsidiary of Berry. As a result of the shareholders of DelMar having a controlling interest in Berry subsequent to the Acquisition, for accounting purposes the transaction constitutes a reverse recapitalization with DelMar being the accounting acquirer even though legally DelMar is the acquiree. Therefore, the net assets of Berry are recorded at fair value at the date of the transaction. No goodwill is recorded with respect to the transaction as it does not constitute a business combination.
In connection with the Exchange Agreement, on January 25, 2013 Berry entered into and closed a series of subscription agreements with accredited investors (the “Investors”), pursuant to which Berry sold an aggregate of 6,704,938 Units at a purchase price of $0.80 per Unit, for aggregate gross proceeds of $5,363,950 (the “Private Offering”). Each Unit consists of one share of common stock and one five-year warrant (the “Investor Warrants”) to purchase one share of common stock at an exercise price of $0.80. The exercise price of the Investor Warrants is subject to adjustment and are redeemable under certain circumstances.
Charles Vista, LLC (the “Placement Agent”) was retained as the placement agent for the Private Offering. The Placement Agent was paid a cash fee of $536,395 (equal to 10% of the gross proceeds), a non-accountable expense allowance of $160,918 (equal to 3% of the gross proceeds), and a consulting fee of $60,000. In addition, the Company issued to the Placement Agent five-year warrants (the “Placement Agent Warrants) to purchase 2,681,975 shares of common stock (equal to 20% of the shares of common stock (i) included as part of the Units sold in the Private Offering and (ii) issuable upon exercise of the Investor Warrants) at an exercise price of $0.80, exercisable on a cash or cashless basis. The Company has agreed to engage the Placement Agent as its warrant solicitation agent in the event the Investor Warrants are called for redemption and will pay a warrant solicitation fee to the Placement Agent equal to 5% of the amount of funds solicited by the Placement Agent upon the exercise of the Investor Warrants following such redemption.
F-44