NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THE TRANSACTION CONTEMPLATED HEREIN; PASSED UPON THE MERITS OR FAIRNESS OF THE TRANSACTION; OR PASSED UPON THE ADEQUACY OR ACCURACY OF THE DISCLOSURE IN THIS DOCUMENT. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
OFFER TO AMEND AND EXERCISE
WARRANTS TO PURCHASE COMMON STOCK
DELMAR PHARMACEUTICALS, INC.
JUNE 9, 2014 (as amended June 26, 2014 and July 10, 2014 )
THE OFFER TO AMEND AND EXERCISE (AND ASSOCIATED WITHDRAWAL RIGHTS) WILL EXPIRE AT 5:00 P.M. (Pacific time) ON JULY 28 , 2014 UNLESS THIS OFFER PERIOD IS EXTENDED.
DelMar Pharmaceuticals, Inc., a Nevada corporation, is referred to in this Offer to Amend and Exercise as “we,” “us,” “DelMar” or the “Company,” and eligible holders of outstanding warrants are referred to as “you.”
The Company is offering to amend, upon the terms and subject to the conditions set forth herein, warrants to purchase an aggregate of 9,195,478 shares of common stock (the “Offer to Amend and Exercise”), including outstanding warrants to purchase 9,195,478 shares of the Company’s common stock issued to investors participating in the Company’s private placement financings closed on January 25, 2013, January 31, 2013, February 8, 2013, February 21, 2013, February 28, 2013, March 1, 2013, and March 6, 2013 (the “ Investor Warrants ”). There is no minimum participation requirement with respect to this Offer to Amend and Exercise.
Pursuant to the Offer to Amend and Exercise, the Investor Warrants will be amended (the “ Amended Warrants ” ) to: (i) reduce the exercise price of the Investor Warrants from $0.80 per share to $0.65 per share of common stock in cash, (ii) shorten the exercise period of the Investor Warrants so that they expire concurrently with the expiration of the Offer to Amend and Exercise at 5:00 p.m. (Pacific Time) on July 28, 2014, as may be extended by the Company in its sole discretion (“ Expiration Date ”), (iii) delete the price-based anti-dilution provisions contained in the Investor Warrants, (iv) restrict the ability of the holder of shares issuable upon exercise of the Amended Warrants to sell, make any short sale of, loan, grant any option for the purchase of, or otherwise dispose of any of such shares without the prior written consent of the Company for a period of time twenty (20) days after the Expiration Date (the “ Lock-Up Period ”); and (v) provide that a holder, acting alone or with others, will agree not to effect any purchases or sales of any securities of the Company in any “short sales” as defined in Rule 200 promulgated under Regulation SHO under the Exchange Act, or any type of direct and indirect stock pledges, forward sale contracts, options, puts, calls, short sales, swaps, “put equivalent positions” (as defined in Rule 16a-1(h) under the Exchange Act) or similar arrangements, or sales or other transactions through non-U.S. broker dealers or foreign regulated brokers through the expiration of the Lock-Up Period. Other than set forth above, the terms of the Investor Warrants will remain unmodified and in full force and effect.
Holders may elect to amend some or all of their Investor Warrants. If you choose not to participate in the Offer to Amend and Exercise, your Investor Warrants will remain in full force and effect, as originally issued.
The purpose of the Offer to Amend and Exercise is to encourage the amendment and exercise of the Investor Warrants to help the Company reduce its outstanding warrant liability and to raise funds to support the Company’s operations by providing the holders of the Investor Warrants with the opportunity to obtain and exercise an Amended Warrant by significantly reducing the exercise price of the Investor Warrants. Please see Section 2 below for a description of the purposes of the Offer to Amend and Exercise.
The period during which Investor Warrants may be amended and exercised on the terms described above will commence on June 9, 2014 (the date the materials relating to the Offer to Amend and Exercise are first sent to the holders, referred to herein as the “Offer Date”) through the Expiration Date (the “Offer Period”).
The Company will agree to amend all Investor Warrants held by eligible holders, upon the terms and subject to the conditions of the Offer to Amend and Exercise and the attached Election to Participate and Exercise Warrant. IT IS THE COMPANY’S CURRENT INTENTION NOT TO CONDUCT ANOTHER OFFER DESIGNED TO INDUCE THE EARLY EXERCISE OF THE INVESTOR WARRANTS.
IMPORTANT PROCEDURES
This Offer to Amend and Exercise together with the Election to Participate and Exercise Warrant, Notice of Withdrawal, and Forms of Amended Warrants constitute the “Offering Materials”. These Offering Materials provide information regarding the Offer to Amend and Exercise and instructions as to how you can amend and exercise your Investor Warrants. An election to participate in the Offer to Amend and Exercise will result in both the amendment of your Investor Warrant(s) and your exercise of the Amended Warrant(s). You should read all of the materials carefully before you decide whether to participate in the Offer to Amend and Exercise an Amended Warrant.
To participate in the Offer to Amend and Exercise accept and exercise an Amended Warrant and receive the number of shares of the Company’s common stock issuable therefor, you must deliver to the Company before the Expiration Date all of the following: (i) a signed copy of the Election to Participate and Exercise Warrant, (ii) a signed copy of an Accredited Investor Questionnaire, (iii) the original copy of your Investor Warrant (or an Affidavit of Lost Warrant) for cancellation, and (iv) cash in the amount equal to $0.65 per share multiplied by the number of shares of common stock the holder elects to purchase (collectively, the “ Acceptance and Exercise Documents ”). The cash may be tendered in the form of a check payable to Signature Bank as Escrow Agent for DelMar Pharmaceuticals, Inc. or by wire transfer to the Company’s escrow account at Signature Bank as set forth in the Election to Participate and Exercise Warrant. The signed copy of the Election to Participate and Exercise Warrant, the signed copy of the Accredited Investor Questionnaire, and the original copy of the Investor Warrant (or an Affidavit of Lost Warrant) for cancellation must be properly delivered, before the Expiration Date to: DelMar Pharmaceuticals, Inc., Suite 720 -- 999 West Broadway, Vancouver, British Columbia CANADA V5Z 1K5, Attn: Corporate Secretary, telephone number (604) 629-5989. If you properly tender (and do not validly withdraw) your Investor Warrants and the other Acceptance and Exercise Documents on or prior to 5:00 p.m. Time on July 28, 2014, the Expiration Date of the Offer to Amend and Exercise (or such later date and time if we extend the Offer to Amend and Exercise), promptly following the Expiration Date, we intend to notify our depositary institution and our transfer agent of our acceptance of your payment of the exercise price and your other Acceptance and Exercise Documents and issue and deliver to you the number of shares of Company common stock issuable under the Amended Warrant . See Section 8 “Procedure for Participating in Offer to Amend and Exercise and Exercising Amended Warrants” below.
If you change your mind and do not want to participate in the Offer to Amend and Exercise, you may submit a Notice of Withdrawal to the Company at any time prior to the Expiration Date The Notice of Withdrawal must be properly completed and must be returned to the Company on or prior to the Expiration Date. However, you may change your mind and submit a Notice of Withdrawal to us after July 28, 2014, if your Investor Warrants and other Acceptance and Exercise Documents have not been accepted by us prior to July 28 , 2014. If you properly withdraw in a timely manner as set forth above, we will promptly: (i) cancel your signed copy of the Election to Participate and Exercise Warrant, (ii) return the original copy of your Investor Warrant (which prior to the Expiration Date, your Investor Warrant will remain unmodified and in full force and effect or issue you a new Investor Warrant if you submitted an Affidavit of Lost Warrant, and (iii) provide you with a check equal to the amount of cash you paid to exercise the Amended Warrant.
If you have any question or need assistance, you should contact National Securities Corporation, (the “Warrant Agent”), for the Offer to Amend and Exercise. The Warrant Agent may be reached at:
National Securities Corporation
Jonathan C. Rich
EVP - Director of Investment Banking
410 Park Avenue 14th Floor
New York, NY. 10022
phone: 212-380-2819
fax: 212-380-2828
You may request additional copies of this document and any of the Offering Materials from the Company. The Company may be reached at:
Suite 720 -- 999 West Broadway
Vancouver, B.C. CANADA V5Z 1K5
Attention: Corporate Secretary
(604) 629-5989
OUR BOARD OF DIRECTORS MAKES NO RECOMMENDATION AS TO WHETHER OR NOT YOU SHOULD PARTICIPATE IN THE OFFER TO AMEND AND EXERCISE. YOU MUST MAKE YOUR OWN DECISION WITH RESPECT TO THE OFFER TO AMEND AND EXERCISE. FOR QUESTIONS REGARDING TAX IMPLICATIONS OR OTHER INVESTMENT-RELATED QUESTIONS, YOU SHOULD TALK TO YOUR OWN ATTORNEY, ACCOUNTANT AND/OR FINANCIAL PLANNER.
WE HAVE NOT AUTHORIZED ANY PERSON TO MAKE ANY RECOMMENDATION ON OUR BEHALF AS TO WHETHER OR NOT YOU SHOULD PARTICIPATE IN THE OFFER TO AMEND AND EXERCISE. YOU SHOULD RELY ONLY ON THE INFORMATION CONTAINED OR INCORPORATED BY REFERENCE IN THIS DOCUMENT.
THIS OFFER TO AMEND AND EXERCISE HAS BEEN PREPARED SOLELY FOR THE BENEFIT OF HOLDERS OF INVESTOR WARRANTS. DISTRIBUTION OF THIS OFFER TO AMEND AND EXERCISE TO ANY PERSON OTHER THAN SUCH HOLDERS AND THOSE PERSONS RETAINED TO ADVISE SUCH HOLDERS IS UNAUTHORIZED AND ANY REPRODUCTION OF THIS OFFER TO AMEND AND EXERCISE OR RELATED DOCUMENTS, IN WHOLE OR IN PART, IS PROHIBITED.
THE SECURITIES BEING OFFERED PURSUANT TO THIS OFFER TO AMEND AND EXERCISE ARE BEING OFFERED PURSUANT TO EXEMPTIONS PROVIDED BY SECTION 4(2) OF THE SECURITIES ACT OF 1933, AS AMENDED, REGULATION D, CERTAIN STATE SECURITIES LAWS AND CERTAIN RULES AND REGULATIONS PROMULGATED THEREUNDER.
THE DATE OF THIS OFFER TO AMEND AND EXERCISE IS JULY 10 , 2014
TABLE OF CONTENTS
| |
|
|
|
|
|
| |
Page
|
| |
|
|
|
|
|
|
1 |
|
| |
|
|
|
|
|
|
6 |
|
| |
|
DESCRIPTION OF OFFER TO AMEND AND EXERCISE
|
|
|
|
15 |
|
| |
|
|
|
|
FORWARD LOOKING STATEMENTS
|
|
|
|
15 |
|
| |
|
|
|
|
PURPOSES OF THE OFFER TO AMEND AND EXERCISE AND USE OF PROCEEDS, PLANS OR PROPOSALS
|
|
|
|
15 |
|
| |
|
|
|
|
ELIGIBILE INVESTOR WARRANTS
|
|
|
|
16 |
|
| |
|
|
|
|
|
|
|
|
16 |
|
| |
|
|
|
|
TERMS OF AMENDED WARRANTS
|
|
|
|
16 |
|
| |
|
|
|
|
CONDITIONS TO THE OFFER TO AMEND AND EXERCISE
|
|
|
|
16 |
|
| |
|
|
|
|
EXTENSION OF OFFER TO AMEND AND EXERCISE PERIOD; TERMINATION; AMENDMENTS
|
|
|
|
17 |
|
| |
|
|
|
|
PROCEDURE FOR PARTICIPATING IN OFFER TO AMEND AND EXERCISE AND EXERCISING AMENDED WARRANTS
|
|
|
|
17 |
|
| |
|
|
|
|
MANNER OF ACCEOPTANCE OF PAYMENT AND ISSUANCE OF SHARES
|
|
|
|
17 |
|
| |
|
|
|
|
|
|
|
|
17 |
|
| |
|
|
|
|
REGISTRATION OF WARRANT SHARES
|
|
|
|
17 |
|
| |
|
|
|
|
TRADING MARKET AND PRICE RANGE OF COMMON STOCK
|
|
|
|
18 |
|
| |
|
|
|
|
SOURCE AND AMOUNT OF FUNDS
|
|
|
|
18 |
|
| |
|
|
|
|
TRANSACTIONS AND AGREEMENTS CONCERNING INVESTOR WARRANTS
|
|
|
|
19 |
|
| |
|
|
|
|
INFORMATION REGARDING THE COMPANY
|
|
|
|
19 |
|
| |
|
|
|
|
HISTORICAL AND PRO-FORMA FINANCIAL INFORMATION REGARDING THE COMPANY
|
|
|
|
26 |
|
| |
|
|
|
|
INTERESTS OF DIRECTORS AND EXECUTIVE OFFICERS IN THE OFFER TO AMEND AND EXERCISE
|
|
|
|
27 |
|
| |
|
|
|
|
LEGAL MATTERS AND REGULATORY APPROVALS
|
|
|
|
27 |
|
| |
|
|
|
|
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES
|
|
|
|
27 |
|
| |
|
|
|
|
|
|
|
|
28 |
|
| |
|
|
|
|
|
|
|
|
29 |
|
| |
|
|
|
|
|
|
|
|
29 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
29 |
|
| |
|
|
|
|
|
|
|
|
HISTORICAL FINANCIAL STATEMENTS FOR THE FISCAL YEARS ENDED DECEMBER 31, 2013 AND 2012
|
|
|
|
A-1
|
|
|
|
HISTORICAL FINANCIAL STATEMENTS FOR THE QUARTERLY PERIOD ENDED MARCH 31, 2014
|
|
|
|
B-1
|
|
| Exhibit C |
PRO FORMA FINANCIAL STATEMENTS |
|
|
|
C-1 |
|
SUMMARY OF TERMS
The Offer to Amend and Exercise is subject to certain conditions, as described herein.
As part of the Election to Participate and Exercise Warrant, the holders of the Investor Warrants must complete an Accredited Investor Questionnaire. In addition, the Company will not accept any Election to Participate and Exercise Warrant from or on behalf of, any Investor Warrant holders if the Company determines that a valid securities exception is not available for the Offer to Amend and Exercise under the Securities Act.
|
Company
|
DelMar Pharmaceuticals, Inc., a Nevada corporation, with principal executive offices at Suite 720 -- 999 West Broadway, Vancouver, British Columbia, CANADA V5Z 1K5. The Company’s telephone number is (604) 629-5989.
|
|
|
Eligible Investor Warrants
|
The following Investor Warrants are subject to the Offer to Amend and Exercise
|
|
|
Investor Warrants
|
Outstanding warrants to purchase 9,195,478 shares of the Company’s common stock issued to investors participating in the Company’s private placement financings closed on January 25, 2013, January 31, 2013, February 8, 2013, February 21, 2013, February 28, 2013, March 1, 2013, and March 6, 2013.
|
|
|
Expiration Date
|
5:00 p.m., Pacific Time on July 28, 2014, as may be extended by the Company in its sole discretion.
|
|
|
Terms of Amended Warrants
|
Pursuant to the Offer to Amend and Exercise, the Investor Warrants will be amended as described below
|
|
|
New Exercise Price
|
The exercise price of the Investor Warrants will be reduced from $0.80 per share to $0.65 per share.
|
|
|
New Termination Date
|
The termination date of the Investor Warrants is being shortened to run concurrently with the Expiration Date.
|
|
|
Lock-Up Period
|
The Amended Warrants will contain a lock-up provision that provides that the holder will not sell, make any short sale of, loan, grant any option for the purchase of, or otherwise dispose of any of the shares issuable upon exercise of the Amended Warrants without the prior written consent of the Company for twenty (20) days after the Expiration Date (the “ Lock-Up Period ”). In addition, the Company may impose stop-transfer restrictions to enforce these restrictions.
|
|
|
No Cashless Exercise
|
The Amended Warrants must be exercised for cash. The shares of common stock issuable upon the exercise of the Amended Warrants will be issued to the holder promptly after the holder’s exercise of the Amended Warrants.
|
|
|
Anti-Dilution
|
The price-based anti-dilution provisions contained in the Investor Warrants will be deleted and will have no application to the issuance (or deemed issuance) or exercise of the Amended Warrants.
|
|
|
Market Restrictions
|
A holder, acting alone or with others, will agree not to effect any purchases or sales of any securities of the Company in any “short sales” as defined in Rule 200 promulgated under Regulation SHO under the Exchange Act, or any type of direct and indirect stock pledges, forward sale contracts, options, puts, calls, short sales, swaps, “put equivalent positions” (as defined in Rule 16a-1(h) under the Exchange Act) or similar arrangements, or sales or other transactions through non-U.S. broker dealers or foreign regulated brokers through the expiration of the Lock-Up Period.
|
|
|
Other Terms
|
Except as set forth above all other terms of the Amended Warrant will be the same as the terms of the Investor Warrants. See the forms of Amended Warrant attached hereto as Exhibit (a)(1)(E) to the Schedule TO.
|
|
|
Partial Participation Permitted
|
If Investor Warrant holders choose to participate in the Offer to Amend and Exercise, they may amend and exercise any or all of such holder’s Investor Warrants pursuant to the terms of the Offer to Amend and Exercise. The Company will issue a new Investor Warrant exercisable for that number of shares of common stock that a holder elects to exclude from the Offer to Amend and Exercise.
|
|
|
Transfers
|
The terms of the Investor Warrants provide that a holder may transfer the Investor Warrants to a third party if the transfer qualifies for an exemption from the registration requirements of the Securities Act to the reasonable satisfaction of the Company. Any holder of an Investor Warrant who desires to transfer an Investor Warrant should contact the Company prior to such transfer to ensure that the planned transfer satisfies the transfer restrictions set forth in the Investor Warrants.
|
|
|
Conditions
|
The Offer to Amend and Exercise is subject to certain conditions, as described herein:
|
|
| |
|
|
| |
(i) As part of the Election to Participate and Exercise Warrant, the holders of the Investor Warrants must complete an Accredited Investor Questionnaire. The holders of the Investor Warrants previously represented to the Company that they were “accredited investors” in connection with the transactions in which such holders acquired the Investor Warrants. The Company has included with this Offer to Amend and Exercise an exhibit titled “Supplemental Company Information” that contains additional information that holders of Investor Warrants who are no longer “accredited investors,” if any, should consider before making an investment decision.
|
|
| |
|
|
| |
(ii) In addition, we are not making this Offer to Amend and Exercise to, nor will we accept any Election to Participate and Exercise Warrant from or on behalf of, Investor Warrant holders in any jurisdiction in which the Offer to Amend and Exercise or the exercise of the Amended Warrants would not be in compliance with the laws of such jurisdiction.
|
|
| |
|
|
| |
You may not elect to amend but not exercise your Investor Warrants. Participation in this Offer to Amend and exercise requires both amendment of your original Investor Warrants and your exercise of the Amended Warrants, which will happen simultaneously should you choose to participate.
|
|
| |
|
|
| |
Investor Warrant holders that elect not to participate and not exercise will remain outstanding pursuant to their original terms.
|
|
| |
|
|
|
Future Amendments to the Offer to Amend and Exercise
|
If we materially change the terms of the Offer to Amend and Exercise we will extend the Expiration Date to the extent required under the rules of the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
|
|
|
|
How to Participate in the Offer to Amend and Exercise
|
To participate in the Offer to Amend and Exercise and exercise an Amended Warrant and receive the number of shares of Company common stock issuable therefor, you must deliver to the Company before the Expiration Date all of the Acceptance and Exercise Documents. The cash exercise price may be tendered in the form of a check payable to Signature Bank as Escrow Agent for DelMar Pharmaceuticals, Inc. or by wire transfer to the Company’s escrow account at Signature Bank as set forth in the Election to Participate and Exercise Warrant. The signed copy of the Election to Participate and Exercise Warrant, the signed copy of the Accredited Investor Questionnaire, and the original copy of the Investor Warrant (or an Affidavit of Lost Warrant) for cancellation must be properly delivered, before the Expiration Date to: DelMar Pharmaceuticals, Inc., Suite 720 -- 999 West Broadway, Vancouver, British Columbia CANADA V5Z 1K5, Attn: Corporate Secretary, telephone number (604) 629-5989.
|
|
|
|
Manner of Acceptance of Payment
|
If you properly tender (and do not validly withdraw) your Investor Warrants and other Acceptance and Exercise Documents on or prior to 5:00 p.m., Pacific Time on July 28, 2014, the Expiration Date of the Offer to Amend and Exercise (or such later date and time if we extend the Offer to Amend and Exercise), promptly following the Expiration Date, we intend to notify our depositary institution and our transfer agent of our acceptance of your payment of the exercise price and your other Acceptance and Exercise Documents and issue and deliver to you the number of shares of Company common stock issuable under the Amended Warrant . See Section 8 “Procedure for Participating in Offer to Amend and Exercise and Exercising Amended Warrants” below.
|
|
|
|
Withdrawal Rights
|
If you change your mind and do not want to participate in the Offer to Amend and Exercise, you may submit the Notice of Withdrawal to us. However, to be effective, the Notice of Withdrawal must be properly completed and must be returned prior to 5:00 p.m., Pacific Time on July 28, 2014, the Expiration Date of the Offer to Amend and Exercise (or such later date and time if we extend the Offer to Amend and Exercise), to: DelMar Pharmaceuticals, Inc., Suite 720 -- 999 West Broadway, Vancouver, British Columbia CANADA V5Z 1K5, Attn: Corporate Secretary, telephone number (604) 629-5989. Following the Expiration Date, you cannot withdraw your Election to Participate and Exercise Warrant. However, if we have not accepted your tendered Investor Warrants and other Acceptance and Exercise Documents by July 28, 2014, which is atleast twenty business day from the commencement of the Offer to Amend and Exercise, you may change your mind and submit a Notice of Withdrawal to us after July 28, 2014.
|
|
|
|
Purposes of the Offer to Amend and Exercise and Use of Proceeds
|
The purposes of this Offer to Amend and Exercise are as follows
|
|
|
|
Reduction of Warrant Liability
|
The Offer to Amend and Exercise can help the Company reduce the warrant liability recorded by the Company on its financial statements. The warrant liability serves as an impediment to certain goals of the Company, as the significant warrant liability on the Company’s balance sheet may make it more difficult for the Company to list its shares of common stock on a national securities exchange. The Investor Warrants contain price-based anti-dilution provisions that provide the holders with protection against future down-round financings. Based on these anti-dilution provisions, the Company is required to record a derivative liability on its balance sheet each fiscal quarter for the Investor Warrants based on the fair value of the Investor Warrants as of the end of such fiscal quarter. The Company’s obligation to continue to record a derivative liability each quarter for a particular Investor Warrant ends when the Investor Warrant is exercised or expires. Various factors are considered in the pricing models the Company used to value the Investor Warrants, including the Company’s current stock price, the remaining life of the Investor Warrants, the volatility of the Company’s stock price, and the risk free interest rate. As a result of the changes in these factors, the derivative warrant liability related solely to the Investor Warrants recorded by the Company was approximately $4,669,731 and $3,511,115 for the periods ended March 31, 2014 and December 31, 2013, respectively. Future changes in these factors will continue to have a significant impact on the computed fair value of the derivative liability for the Investor Warrants. As such, the Company expects future changes in the fair value of the Investor Warrants to continue to vary significantly from quarter to quarter. The Company believes these significant variations make it more difficult for investors to evaluate the Company’s business and operations.
|
|
|
|
Fund Raising
|
An additional purpose of the Offer to Amend and Exercise is to raise funds to support the Company’s future operations and capital requirements by encouraging the participating holders to exercise their Investor Warrants by significantly reducing the exercise price and shortening the exercise period. The funds obtained will be used by the Company as working capital and for other general corporate purposes.
|
|
|
|
Registration of Warrant Shares
|
The shares of common stock issuable upon exercise of the Amended Warrants are “restricted securities” and may not be sold by the holder absent a registration statement covering the resale of the shares or an exemption from the registration requirement. We have previously filed a Registration Statement on Form S-1 to register the resale of the shares of common stock underlying the Investor Warrants under the Securities Act. Promptly following the Expiration Date, we intend to file a prospectus supplement to the prospectus included in the Registration Statement to reflect the substantive changes from the information currently set forth in such prospectus as a result of the Offer to Amend and Exercise Thereafter, the holders of shares of common stock issuable upon exercise of the Amended Warrants who are listed as selling stockholders in the Registration Statement may sell their shares of common stock in accordance with the resale restrictions set forth in the “Plan of Distribution” section of the Prospectus in the Registration Statement. Each holder of Investor Warrants should read the applicable Prospectus carefully before deciding whether to participate in the Offer to Amend and Exercise. In addition, any holder (including any transferees or acquirers) of an Investor Warrant or Amended Warrant who is not listed as a selling stockholder in the Prospectus cannot resell such holder’s shares in reliance on the Prospectus, unless and until the Company files a post-effective amendment to the Registration Statement to include such holder as a selling stockholder. Absent the filing of the post-effective amendment to the Registration Statement, the holder (including any transferees or acquirers) will be required to qualify for an exemption from the registration requirements, which may require a holding period of at least six months.
|
|
|
|
Taxes
|
We recommend that you consult with your own tax advisor with regard to the possibility of any federal, state, local or other tax consequences of the Offer to Amend and Exercise. See Section 19 “Material U.S. Federal Income Tax Consequences” below for a discussion of the material U.S. Federal Income Tax Consequences of participating in the Offer to Amend and Exercise.
|
|
|
|
Fees and Expenses
|
Commercial efforts to contact holders of the Investor Warrants by mail, telephone, facsimile, or other electronic means and solicit their participation in the Offer to Amend and Exercise. National Securities Corporation will receive a fee equal to 5% of the cash exercise prices paid by holders of the Investor Warrants who participate in the Offer to Amend and Exercise. In addition, the Company has agreed to reimburse National Securities Corporation for its reasonable out-of-pocket expenses. If such expenses and fees exceed $1,000, National Securities Corporation must obtain the Company’s prior approval. We have also issued National Securities Corporation a warrant to purchase 300,000 shares of the Company’s common stock at an exercise price of $1.76 per share. The warrant terminates on August 15, 2018. The Company has agreed to indemnify National Securities Corporation against certain liabilities in connection with the Offer to Amend and Exercise, including certain liabilities under the federal securities laws.
|
|
|
|
Interests of Directors and Executive Officers
|
None of our executive officers or directors hold Investor Warrants. Please see Section 17 “Interests of Directors and Officers in the Offer to Amend and Exercise” below.
|
|
|
Additional Information
|
The Board of Directors of the Company recognizes that the decision to participate in the Offer to Amend and Exercise is an individual one that should be based on a variety of factors. The holders of the Investor Warrants should consult with their respective professional advisors if they have questions about their financial or tax situation. The information about this Offer to Amend and Exercise from the Company is limited to the Offering Materials. The Company is subject to the information requirements of the Securities Exchange Act of 1934, as amended, and in accordance therewith files and furnishes reports and other information with the SEC. All reports and other documents the Company has filed with the SEC, including the Schedule TO relating to the Offer to Amend and Exercise, or will file with the SEC in the future, can be accessed electronically on the SEC’s website at www.sec.gov.
|
|
|
Information Requests
|
Please direct questions or requests for assistance regarding this Offer to Amend and Exercise, Election to Participate and Exercise Warrant, and Notice of Withdrawal or other materials, in writing, to the Warrant Agent:
Jonathan C. Rich
EVP - Director of Investment Banking
National Securities Corporation
410 Park Avenue 14th Floor
New York, NY. 10022
phone: 212-380-2819
fax: 212-380-2828
|
|
Please direct requests for additional copies of this Offer to Amend and Exercise, Election to Participate and Exercise Warrant, and Notice of Withdrawal or other materials, in writing, to the Company — DelMar Pharmaceuticals, Inc., Suite 720 - 999 West Broadway, Vancouver, British Columbia CANADA V5Z 1K5; Attn: Corporate Secretary, telephone (604) 629-5989.
ABOUT THIS OFFER TO AMEND AND EXERCISE
YOU SHOULD RELY ONLY ON THE INFORMATION CONTAINED OR INCORPORATED BY REFERENCE IN THIS OFFER TO AMEND AND EXERCISE. WE HAVE NOT AUTHORIZED ANYONE TO PROVIDE INFORMATION DIFFERENT FROM THAT CONTAINED OR INCORPORATED BY REFERENCE IN THIS OFFER TO AMEND AND EXERCISE AND, IF PROVIDED, SUCH INFORMATION MUST NOT BE RELIED UPON.
ALTHOUGH OUR BOARD OF DIRECTORS HAS APPROVED THE OFFER TO AMEND AND EXERCISE, NEITHER THE COMPANY, ITS DIRECTORS, OFFICERS, ADVISORS OR AGENTS, INCLUDING THE WARRANT AGENT, MAKES ANY RECOMMENDATION AS TO WHETHER YOU SHOULD ACCEPT THE OFFER TO AMEND AND EXERCISE. YOU SHOULD NOT CONSIDER THE BOARD’S APPROVAL TO BE A RECOMMENDATION AS TO WHETHER YOU SHOULD PARTICIPATE IN THE OFFER TO AMEND AND EXERCISE WARRANTS. YOU MUST MAKE YOUR OWN DECISION WHETHER TO ACCEPT THE OFFER TO AMEND AND EXERCISE.
RISK FACTORS
Investment in our common stock involves a substantial degree of risk and should be regarded as speculative. As a result, the purchase of our common stock should be considered only by persons who can reasonably afford to lose their entire investment. Before you elect to participate in the Offer to Amend and Exercise, you should carefully consider the risk and uncertainties described below in addition to the other information in this Offer to Amend and Exercise and other information incorporated herein by reference. Additional risks and uncertainties of which we are unaware or which we currently believe are immaterial could also materially adversely affect our business, financial condition or results of operations. In any case, the trading price of our common stock could decline, and you could lose all or part of your investment.
Risks related to our Business and our Industry
We have a limited operating history and a history of operating losses, and expect to incur significant additional operating losses.
We are a development stage company. Our subsidiary, Del Mar Pharmaceuticals (BC) Ltd. (“DelMar (BC)”) was incorporated in British Columbia on April 6, 2010 and has only a limited operating history. Therefore, there is limited historical financial information upon which to base an evaluation of our performance. Our prospects must be considered in light of the uncertainties, risks, expenses, and difficulties frequently encountered by companies in their early stages of operations. We have generated net losses since we began operations, including $8,290,689 and $2,400,363 for the years ended December 31, 2013 and 2012, respectively. We expect to incur substantial additional net expenses over the next several years as our research, development, and commercial activities increase. The amount of future losses and when, if ever, we will achieve profitability are uncertain. Our ability to generate revenue and achieve profitability will depend on, among other things, successful completion of the preclinical and clinical development of our product candidates; obtaining necessary regulatory approvals from the FDA and international regulatory agencies; successful manufacturing, sales, and marketing arrangements; and raising sufficient funds to finance our activities. If we are unsuccessful at some or all of these undertakings, our business, prospects, and results of operations may be materially adversely affected.
Our independent auditor has expressed substantial doubt about our ability to continue as a going concern. Our ability to continue is dependent on our ability to raise additional capital and our operations could be curtailed if we are unable to obtain the required additional funding when needed.
There is a large degree of uncertainty as to the expenses the Company will incur in developing and pursuing its business plan. In addition, the Company has not begun to generate revenues. Consequently, our audited financial statements for the fiscal year ended December 31, 2013, include an explanatory paragraph that such financial statements were prepared assuming that we would continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Consequently, in the future management will need to pursue various financing alternatives to fund the Company’s operations so it can continue as a going concern in the medium to longer term. Accordingly, the Company is considered to be in the development stage as defined in Accounting Standards Codification (ASC) 915-10. We believe, based on our current estimates and plans we expect to have enough cash to fund our operations for the next 12 to 15 months. Management plans to secure the necessary financing through the issue of new equity and/or the entering into of strategic partnership arrangements. Nevertheless, there is no assurance that these initiatives will be successful.
There could be material differences in our cost estimates or there can be unforeseen events, problems or delays will occur that would require us to seek additional debt and/or equity funding. The ability of the Company to meet its obligations and continue the research and development of its product candidate is dependent on its ability to continue to raise adequate financing. There can be no assurance that such financing will be available to the Company in the amount required at any time or for any period or, if available, that it can be obtained on terms satisfactory to the Company. The Company may tailor its drug candidate program based on the amount of funding it raises.
Our future funding requirements will depend on many factors, including but not limited to:
| ● |
|
the rate of progress and cost of our clinical trials, preclinical studies and other discovery and research and development activities; |
| |
|
|
| ● |
|
the costs associated with establishing manufacturing and commercialization capabilities; |
| |
|
|
| ● |
|
the costs of acquiring or investing in businesses, product candidates and technologies; |
| |
|
|
| ● |
|
the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
| |
|
|
| ● |
|
the costs and timing of seeking and obtaining FDA and other regulatory approvals; |
| |
|
|
| ● |
|
the effect of competing technological and market developments; and |
| |
|
|
| ● |
|
the economic and other terms and timing of any collaboration, licensing or other arrangements into which we may enter. |
Until we can generate a sufficient amount of product revenue to finance our cash requirements, which we may never do, we expect to finance future cash needs primarily through public or private equity offerings, debt financings or strategic collaborations. Although we are not reliant on institutional credit finance and therefore not subject to debt covenant compliance requirements or potential withdrawal of credit by banks, the current economic climate has also impacted the availability of funds and activity in equity markets. We do not know whether additional funding will be available on acceptable terms, or at all. If we are not able to secure additional funding when needed, we may have to delay, reduce the scope of or eliminate one or more of our clinical trials or research and development programs or make changes to our operating plan. In addition, we may have to establish collaborations, partnerships, or license our product programs at an early stage of development, which would lower the economic value of those programs to us.
We will need to secure additional financing.
We anticipate that we will incur operating losses for the foreseeable future. We will require additional funds for our anticipated operations and if we are not successful in securing additional financing, we may be required to delay significantly, reduce the scope of our research and development program, downsize our general and administrative infrastructure, or seek alternative measures to avoid insolvency, including arrangements with collaborative partners or others that may require us to relinquish rights to certain of our technologies or product candidate.
We are an early-stage company with an unproven business strategy and may never achieve commercialization of our candidate products or profitability.
We are at an early stage of development and commercialization of our technologies and product candidates. We have not yet begun to market any products and, accordingly, have not begun or generate revenues from the commercialization of our product. Our product will require significant additional clinical testing and investment prior to commercialization. A commitment of substantial resources by ourselves and, potentially, our partners to conduct time-consuming research and clinical trials will be required if we are to complete the development of our product candidate. There can be no assurance that our product candidate will meet applicable regulatory standards, obtain required regulatory approvals, be capable of being produced in commercial quantities at reasonable costs or be successfully marketed. Our product candidate is not expected to be commercially available for several years, if at all.
We are currently focused on the development of a single product candidate
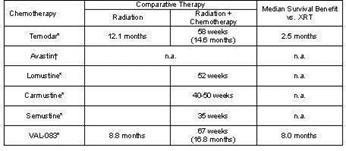
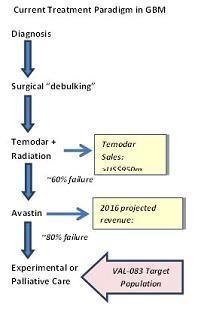
Our product development efforts are currently focused on a single product, VAL-083, for which we are researching multiple indications. If VAL-083 fails to achieve clinical endpoints or exhibits unanticipated toxicity or if a superior product is developed by a competitor, our prospects for obtaining regulatory approval and commercialization may be negatively impacted. In the long term, we hope to establish a pipeline of product candidates and we have identified additional product candidates that we may be able to acquire or license in the future. However, at this time we do not have any formal agreements granting us any rights to such additional product candidates.
Our collaborators’ ability to sell therapeutic products will depend to a large extent upon reimbursement from health care insurance companies.
Our success may depend, in part, on the extent to which reimbursement for the costs of therapeutic products and related treatments will be available from third-party payers such as government health administration authorities, private health insurers, managed care programs, and other organizations. Over the past decade, the cost of health care has risen significantly, and there have been numerous proposals by legislators, regulators, and third-party health care payers to curb these costs. Some of these proposals have involved limitations on the amount of reimbursement for certain products. Similar federal or state health care legislation may be adopted in the future and any products that we or our collaborators seek to commercialize may not be considered cost-effective. Adequate third-party insurance coverage may not be available for us or our collaborative partners to establish and maintain price levels that are sufficient for realization of an appropriate return on investment in product development.
We are dependent on obtaining certain patents and protecting our proprietary rights.
Our success will depend, in part, on our ability to obtain patents, maintain trade secret protection and operate without infringing on the proprietary rights of third parties or having third parties circumvent our rights. We have filed and are actively pursuing patent applications for our products. The patent positions of biotechnology, biopharmaceutical and pharmaceutical companies can be highly uncertain and involve complex legal and factual questions. Thus, there can be no assurance that any of our patent applications will result in the issuance of patents, that we will develop additional proprietary products that are patentable, that any patents issued to us or those that already have been issued will provide us with any competitive advantages or will not be challenged by any third parties, that the patents of others will not impede our ability to do business or that third parties will not be able to circumvent our patents. Furthermore, there can be no assurance that others will not independently develop similar products, duplicate any of our products not under patent protection, or, if patents are issued to us, design around the patented products we developed or will develop.
We may be required to obtain licenses from third parties to avoid infringing patents or other proprietary rights. No assurance can be given that any licenses required under any such patents or proprietary rights would be made available, if at all, on terms we find acceptable. If we do not obtain such licenses, we could encounter delays in the introduction of products, or could find that the development, manufacture or sale of products requiring such licenses could be prohibited.
A number of pharmaceutical, biopharmaceutical and biotechnology companies and research and academic institutions have developed technologies, filed patent applications or received patents on various technologies that may be related to or affect our business. Some of these technologies, applications or patents may conflict with our technologies or patent applications. Such conflict could limit the scope of the patents, if any, that we may be able to obtain or result in the denial of our patent applications. In addition, if patents that cover our activities are issued to other companies, there can be no assurance that we would be able to obtain licenses to these patents at a reasonable cost or be able to develop or obtain alternative technology. If we do not obtain such licenses, we could encounter delays in the introduction of products, or could find that the development, manufacture or sale of products requiring such licenses could be prohibited. In addition, we could incur substantial costs in defending ourselves in suits brought against us on patents it might infringe or in filing suits against others to have such patents declared invalid.
Patent applications in the U.S. are maintained in secrecy and not published if either: i) the application is a provisional application or, ii) the application is filed and we request no publication, and certify that the invention disclosed “has not and will not” be the subject of a published foreign application. Otherwise, U.S. applications or foreign counterparts, if any, publish 18 months after the priority application has been filed. Since publication of discoveries in the scientific or patent literature often lag behind actual discoveries, we cannot be certain that we or any licensor were the first creator of inventions covered by pending patent applications or that we or such licensor was the first to file patent applications for such inventions. Moreover, we might have to participate in interference proceedings declared by the U.S. Patent and Trademark Office to determine priority of invention, which could result in substantial cost to us, even if the eventual outcome were favorable to us. There can be no assurance that our patents, if issued, would be held valid or enforceable by a court or that a competitor’s technology or product would be found to infringe such patents.
In addition, the protection of intellectual property rights in China (where our lead product candidate, VAL-083, is manufactured pursuant to a collaboration agreement with the only manufacturer presently licensed by the CFDA to produce the product for the China market, and where VAL-083 is approved for the treatment of CML and lung cancer) is relatively weak compared to the United States, which may negatively affect our ability to generate revenue from VAL-083.
Much of our know-how and technology may not be patentable. To protect our rights, we require employees, consultants, advisors and collaborators to enter into confidentiality agreements. There can be no assurance, however, that these agreements will provide meaningful protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure. Further, our business may be adversely affected by competitors who independently develop competing technologies, especially if we obtain no, or only narrow, patent protection.
We are subject to various government regulations.
The manufacture and sale of human therapeutic and diagnostic products in the U.S., Canada and foreign jurisdictions are governed by a variety of statutes and regulations. These laws require approval of manufacturing facilities, controlled research and testing of products and government review and approval of a submission containing manufacturing, preclinical and clinical data in order to obtain marketing approval based on establishing the safety and efficacy of the product for each use sought, including adherence to current cGMP during production and storage, and control of marketing activities, including advertising and labeling.
The product we are currently developing will require significant development, preclinical and clinical testing and investment of substantial funds prior to their commercialization. The process of obtaining required approvals can be costly and time-consuming, and there can be no assurance that future products will be successfully developed and will prove to be safe and effective in clinical trials or receive applicable regulatory approvals. Markets other than the U.S. and Canada have similar restrictions. Potential investors and shareholders should be aware of the risks, problems, delays, expenses and difficulties which we may encounter in view of the extensive regulatory environment which controls our business.
If we are unable to keep up with rapid technological changes in our field or compete effectively, we will be unable to operate profitably.
We are engaged in a rapidly changing field. Other products and therapies that will compete directly with the products that we are seeking to develop and market currently exist or are being developed. Competition from fully integrated pharmaceutical companies and more established biotechnology companies is intense and is expected to increase. Most of these companies have significantly greater financial resources and expertise in discovery and development, manufacturing, preclinical and clinical testing, obtaining regulatory approvals and marketing than us. Smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large pharmaceutical and established biopharmaceutical or biotechnology companies. Many of these competitors have significant products that have been approved or are in development and operate large, well-funded discovery and development programs. Academic institutions, governmental agencies and other public and private research organizations also conduct research, seek patent protection and establish collaborative arrangements for therapeutic products and clinical development and marketing. These companies and institutions compete with us in recruiting and retaining highly qualified scientific and management personnel. In addition to the above factors, we will face competition based on product efficacy and safety, the timing and scope of regulatory approvals, availability of supply, marketing and sales capability, reimbursement coverage, price and patent position. There is no assurance that our competitors will not develop more effective or more affordable products, or achieve earlier patent protection or product commercialization, than our own.
Other companies may succeed in developing products earlier than ourselves, obtaining Health Canada, EMA and FDA approvals for such products more rapidly than we will, or in developing products that are more effective than products we propose to develop. While we will seek to expand our technological capabilities in order to remain competitive, there can be no assurance that research and development by others will not render our technology or products obsolete or non-competitive or result in treatments or cures superior to any therapy we develop, or that any therapy we develop will be preferred to any existing or newly developed technologies.
Clinical trials for our product candidate are expensive and time consuming, and their outcome is uncertain.
The process of obtaining and maintaining regulatory approvals for new therapeutic product is expensive, lengthy and uncertain. Costs and timing of clinical trials may vary significantly over the life of a project owing to any or all of the following non-exclusive reasons:
| ● |
|
the duration of the clinical trial; |
| |
|
|
| ● |
|
the number of sites included in the trials; |
| |
|
|
| ● |
|
the countries in which the trial is conducted; |
| |
|
|
| ● |
|
the length of time required and ability to enroll eligible patients; |
| |
|
|
| ● |
|
the number of patients that participate in the trials; |
| |
|
|
| ● |
|
the number of doses that patients receive; |
| |
|
|
| ● |
|
the drop-out or discontinuation rates of patients;
|
| |
|
|
| ● |
|
per patient trial costs; |
| |
|
|
| ● |
|
third party contractors failing to comply with regulatory requirements or meet their contractual obligations to us in a timely manner; |
| |
|
|
| ● |
|
our final product candidates having different properties in humans than in laboratory testing; |
| |
|
|
| ● |
|
the need to suspect or terminate our clinical trials; |
| |
|
|
| ● |
|
insufficient or inadequate supply of quality of necessary materials to conduct our trials; |
| |
|
|
| ● |
|
potential additional safety monitoring, or other conditions required by FDA or comparable foreign regulatory authorities regarding the scope or design of our clinical trials, or other studies requested by regulatory agencies; |
| |
|
|
| ● |
|
problems engaging institutional review boards, or IRBs, to oversee trials or in obtaining and maintaining IRB approval of studies; |
| |
|
|
| ● |
|
the duration of patient follow-up; |
| |
|
|
| ● |
|
the efficacy and safety profile of a product candidate; |
| |
|
|
| ● |
|
the costs and timing of obtaining regulatory approvals; and |
| |
|
|
| ● |
|
the costs involved in enforcing or defending patent claims or other intellectual property rights. |
Late stage clinical trials are especially expensive, typically requiring tens of millions of dollars, and take years to reach their outcomes. Such outcomes often fail to reproduce the results of earlier trials. It is often necessary to conduct multiple late stage trials (including multiple Phase III trials) in order to obtain sufficient results to support product approval, which further increases the expense. Sometimes trials are further complicated by changes in requirements while the trials are under way (for example, when the standard of care changes for the disease that is being studied in the trial). Accordingly, any of our current or future product candidates could take a significantly longer time to gain regulatory approval than we expect, or may never gain approval, either of which could delay or stop the commercialization of our product candidates.
We may be required to suspend or discontinue clinical trials due to unexpected side effects or other safety risks that could preclude approval of our product candidates.
Our clinical trials may be suspended at any time for a number of reasons. For example, we may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to the clinical trial patients. In addition, the FDA or other regulatory agencies may order the temporary or permanent discontinuation of our clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to the clinical trial patients.
Administering any product candidate to humans may produce undesirable side effects. These side effects could interrupt, delay or halt clinical trials of our product candidates and could result in the FDA or other regulatory authorities denying further development or approval of our product candidates for any or all targeted indications. Ultimately, some or all of our product candidates may prove to be unsafe for human use. Moreover, we could be subject to significant liability if any volunteer or patient suffers, or appears to suffer, adverse health effects as a result of participating in our clinical trials.
We may not receive regulatory approvals for our product candidate or there may be a delay in obtaining such approvals.
Our product and our ongoing development activities are subject to regulation by regulatory authorities in the countries in which we or our collaborators and distributors wish to test, manufacture or market our products. For instance, the FDA will regulate the product in the U.S. and equivalent authorities, such as the EMA, will regulate in Europe. Regulatory approval by these authorities will be subject to the evaluation of data relating to the quality, efficacy and safety of the product for its proposed use, and there can be no assurance that the regulatory authorities will find our data sufficient to support product approval of VAL-083.
The time required to obtain regulatory approval varies between countries. In the U.S., for products without “Fast Track” status, it can take up to eighteen (18) months after submission of an application for product approval to receive the FDA's decision. Even with Fast Track status, FDA review and decision can take up to twelve (12) months. At present, we do not have Fast Track status for our lead product candidate, VAL-083.
Different regulators may impose their own requirements and may refuse to grant, or may require additional data before granting, an approval, notwithstanding that regulatory approval may have been granted by other regulators. Regulatory approval may be delayed, limited or denied for a number of reasons, including insufficient clinical data, the product not meeting safety or efficacy requirements or any relevant manufacturing processes or facilities not meeting applicable requirements as well as case load at the regulatory agency at the time.
We may fail to comply with regulatory requirements.
Our success will be dependent upon our ability, and our collaborative partners’ abilities, to maintain compliance with regulatory requirements, including cGMP, and safety reporting obligations. The failure to comply with applicable regulatory requirements can result in, among other things, fines, injunctions, civil penalties, total or partial suspension of regulatory approvals, refusal to approve pending applications, recalls or seizures of products, operating and production restrictions and criminal prosecutions.
Regulatory approval of our products may be withdrawn at any time.
After regulatory approval has been obtained for medicinal products, the product and the manufacturer are subject to continual review, including the review of adverse experiences and clinical results that are reported after our products are made available to patients, and there can be no assurance that such approval will not be withdrawn or restricted. Regulators may also subject approvals to restrictions or conditions, or impose post-approval obligations on the holders of these approvals, and the regulatory status of such products may be jeopardized if such obligations are not fulfilled. If post-approval studies are required, such studies may involve significant time and expense.
The manufacturer and manufacturing facilities we use to make any of our products will also be subject to periodic review and inspection by the FDA or EMA, as applicable. The discovery of any new or previously unknown problems with the product, manufacturer or facility may result in restrictions on the product or manufacturer or facility, including withdrawal of the product from the market. We will continue to be subject to the FDA or EMA requirements, as applicable, governing the labeling, packaging, storage, advertising, promotion, recordkeeping, and submission of safety and other post-market information for all of our product candidates, even those that the FDA or EMA, as applicable, had approved. If we fail to comply with applicable continuing regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory approval, product recalls and seizures, operating restrictions and other adverse consequences.
We manufacture our clinical supplies at a single location. Any disruption at this facility could adversely affect our business and results of operations.
We rely on our manufacturing partner, Guangxi Wuzhou Pharmaceuticals (Group) Co. Ltd. for the manufacture of clinical supply of VAL-083. If our partner’s facility were damaged or destroyed, or otherwise subject to disruption, it would require substantial lead-time to replace our clinical supply. In such event, we would be forced to rely entirely on other third-party contract manufacturers for an indefinite period of time. We have established a relationship with a back-up manufacturer, which has produced quantities of the active pharmaceutical ingredient contained in VAL-083. However, at this time no drug product has been manufactured by a third-party back-up manufacturer. Any disruptions or delays by Guangxi Wuzhou Pharmaceuticals or their failure to meet regulatory compliance could impair our ability to develop VAL-083, which would adversely affect our business and results of operations.
There may not be a viable market for our product.
We believe that there will be many different applications for our product. We also believe that the anticipated market for our product will continue to expand. These assumptions may prove to be incorrect for a variety of reasons, including competition from other products and the degree of our products’ commercial viability.
We rely on key personnel and, if we are unable to retain or motivate key personnel or hire qualified personnel, we may not be able to grow effectively.
We are dependent on certain members of our management, scientific and drug development staff and consultants, the loss of services of one or more of whom could materially adversely affect us.
We currently have four full-time employees, and retain the services of approximately 19 persons on an independent contractor/consultant and contract-employment basis. Our ability to manage growth effectively will require us to continue to implement and improve our management systems and to recruit and train new employees. Although we have done so in the past and expect to do so in the future, there can be no assurance that we will be able to successfully attract and retain skilled and experienced personnel.
We may be subject to foreign exchange fluctuation.
Our functional and reporting currency is the United States dollar. We maintain bank accounts in United States and Canadian dollars. A portion of our expenditures are in foreign currencies, most notably in Canadian dollars, and therefore we are subject to foreign currency fluctuations, which may, from time to time, impact our financial position and results. We may enter into hedging arrangements under specific circumstances, typically through the use of forward or futures currency contracts, to minimize the impact of increases in the value of the Canadian dollar. In order to minimize our exposure to foreign exchange fluctuations we may hold sufficient Canadian dollars to cover our expected Canadian dollar expenditures.
We may be exposed to potential product and clinical trials liability.
Our business exposes us to potential product liability risks, which are inherent in the testing, manufacturing, marketing and sale of therapeutic products. Human therapeutic products involve an inherent risk of product liability claims and associated adverse publicity. While we will continue to take precautions we deem appropriate, there can be no assurance that we will be able to avoid significant product liability exposure. We maintain liability insurance coverage. Such insurance is expensive, difficult to obtain and may not continue to be available on acceptable terms, if at all. An inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of our current or potential products. A product liability claim brought against us in a clinical trial or a product withdrawal could have a material adverse effect upon us and our financial condition.
We are dependent on our collaborative partners and service providers the loss of which would hurt our business.
Our strategy is to enter into various arrangements with corporate and academic collaborators, licensors, licensees, service providers and others for the research, development, clinical testing and commercialization of our product. We intend to or have entered into agreements with academic, medical and commercial organizations to research, develop and test our product. In addition, we intend to enter into corporate partnerships to commercialize the Company’s core product. There can be no assurance that such collaborations can be established on favorable terms, if at all.
Should any collaborative partner or service provider fail to appropriately research, develop, test or successfully commercialize any product to which the Company has rights, our business may be adversely affected. Failure of a collaborative partner or service provider to successfully conduct or complete their activities or to remain a viable collaborative partner or commercialize enterprise for any particular program could delay or halt the development or commercialization of any products arising out of such program. While management believes that collaborative partners and service providers will have sufficient economic motivation to continue their activities, there can be no assurance that any of these collaborations or provisions of required services will be continued or result in successfully commercialized products.
In addition, there can be no assurance that the collaborative research or commercialization partners will not pursue alternative technologies or develop alternative products either on their own or in collaboration with others, including our competitors, as a means for developing treatments for the diseases or conditions targeted by our programs.
We may become subject to liabilities related to risks inherent in working with hazardous materials.
Our discovery and development processes involve the controlled use of hazardous and radioactive materials. We are subject to federal, provincial and local laws and regulations governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by such laws and regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result and any such liability could exceed our resources. We are not specifically insured with respect to this liability. Although we believe that we are in compliance in all material respects with applicable environmental laws and regulations and currently do not expect to make material capital expenditures for environmental control facilities in the near-term, there can be no assurance that we will not be required to incur significant costs to comply with environmental laws and regulations in the future, or that our operations, business or assets will not be materially adversely affected by current or future environmental laws or regulations.
Risks Related to Our Common Stock
The Company has a limited trading history , and you may have difficulty trading and obtaining quotations for our common stock.
The Company’s common stock is registered under the Exchange Act and is quoted on the OTC Bulletin Board. Prior to January 25, 2013, there was no reported trading in the Company’s common stock. Since January 25, 2013, there has been limited trading in our common stock. As a result, investors may find it difficult to dispose of, or to obtain accurate quotations of the price of, our securities. This severely limits the liquidity of the common stock, and may adversely affect the market price of our common stock. A limited market may also impair our ability to raise capital by selling shares of capital stock and may impair our ability to acquire other companies or assets by using common stock as consideration. Further, there is no established trading market for the Investor Warrants nor will there ever be an established trading market for the Amended Warrants.
The market price of our common stock is, and is likely to continue to be, highly volatile and subject to wide fluctuations.
The market price of our common stock is highly volatile and could be subject to wide fluctuations in response to a number of factors that are beyond our control, including:
| ● |
|
variations in our quarterly operating results; |
| |
|
|
| ● |
|
announcements that our revenue or income are below analysts’ expectations; |
| |
|
|
| ● |
|
general economic slowdowns; |
| |
|
|
| ● |
|
sales of large blocks of the Company’s common stock; and |
| |
|
|
| ● |
|
announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments. |
Our common stock is subject to the “penny stock” rules of the Securities and Exchange Commission, which may make it more difficult for stockholders to sell our common stock.
The SEC has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require that a broker or dealer approve a person’s account for transactions in penny stocks, and the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must obtain financial information and investment experience objectives of the person, and make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form sets forth the basis on which the broker or dealer made the suitability determination, and that the broker or dealer received a signed, written agreement from the investor prior to the transaction.
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of the Company’s common stock if and when such shares are eligible for sale and may cause a decline in the market value of its stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stock.
Because we became public by means of a reverse acquisition, we may not be able to attract the attention of brokerage firms.
Because we became public through a “reverse acquisition”, securities analysts of brokerage firms may not provide coverage of us since there is little incentive to brokerage firms to recommend the purchase of our common stock. No assurance can be given that brokerage firms will want to conduct any secondary offerings on behalf of the Company in the future.
Applicable regulatory requirements, including those contained in and issued under the Sarbanes-Oxley Act of 2002, may make it difficult for the Company to retain or attract qualified officers and directors, which could adversely affect the management of its business and its ability to obtain or retain listing of its common stock.
The Company may be unable to attract and retain those qualified officers, directors and members of board committees required to provide for effective management because of the rules and regulations that govern publicly held companies, including, but not limited to, certifications by principal executive officers. The enactment of the Sarbanes-Oxley Act has resulted in the issuance of a series of related rules and regulations and the strengthening of existing rules and regulations by the SEC, as well as the adoption of new and more stringent rules by the stock exchanges. The perceived increased personal risk associated with these changes may deter qualified individuals from accepting roles as directors and executive officers.
Further, some of these changes heighten the requirements for board or committee membership, particularly with respect to an individual’s independence from the corporation and level of experience in finance and accounting matters. The Company may have difficulty attracting and retaining directors with the requisite qualifications. If the Company is unable to attract and retain qualified officers and directors, the management of its business and its ability to obtain or retain listing of our shares of common stock on any stock exchange (assuming the Company elects to seek and are successful in obtaining such listing) could be adversely affected.
If the Company fails to maintain an effective system of internal controls, it may not be able to accurately report its financial results or detect fraud. Consequently, investors could lose confidence in the Company’s financial reporting and this may decrease the trading price of its stock.
The Company must maintain effective internal controls to provide reliable financial reports and detect fraud. The Company has been assessing its internal controls to identify areas that need improvement. It is in the process of implementing changes to internal controls, but has not yet completed implementing these changes. Failure to implement these changes to the Company’s internal controls or any others that it identifies as necessary to maintain an effective system of internal controls could harm its operating results and cause investors to lose confidence in the Company’s reported financial information. Any such loss of confidence would have a negative effect on the trading price of the Company’s stock.
Voting power of our shareholders is highly concentrated by insiders.
Our officers and directors control, either directly or indirectly, a substantial portion of our voting securities. Therefore, our management may significantly affect the outcome of all corporate actions and decisions for an indefinite period of time including election of directors, amendment of charter documents and approval of mergers and other significant corporate transactions.
We do not intend to pay dividends for the foreseeable future.
We have paid no dividends on our common stock to date and it is not anticipated that any dividends will be paid to holders of our common stock in the foreseeable future. While our future dividend policy will be based on the operating results and capital needs of the business, it is currently anticipated that any earnings will be retained to finance our future expansion and for the implementation of our business plan. As an investor, you should take note of the fact that a lack of a dividend can further affect the market value of our common stock, and could significantly affect the value of any investment in our Company.
Our articles of incorporation allow for our board to create new series of preferred stock without further approval by our stockholders, which could adversely affect the rights of the holders of our common stock.
Our Board of Directors has the authority to fix and determine the relative rights and preferences of preferred stock. Our Board of Directors has the authority to issue up to 5,000,000 shares of our preferred stock (of which 1 share has been designated Special Voting Preferred Stock and is issued and outstanding) without further stockholder approval. As a result, our Board of Directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock and the right to the redemption of the shares, together with a premium, prior to the redemption of our common stock. In addition, our board of directors could authorize the issuance of a series of preferred stock that has greater voting power than our common stock or that is convertible into our common stock, which could decrease the relative voting power of our common stock or result in dilution to our existing stockholders. Although we have no present intention to issue any additional shares of preferred stock or to create any additional series of preferred stock, we may issue such shares in the future.
As an issuer of “penny stock”, the protection provided by the federal securities laws relating to forward looking statements does not apply to us.
Although federal securities laws provide a safe harbor for forward-looking statements made by a public company that files reports under the federal securities laws, this safe harbor is not available to issuers of penny stocks. As a result, we will not have the benefit of this safe harbor protection in the event of any legal action based upon a claim that the material provided by us contained a material misstatement of fact or was misleading in any material respect because of our failure to include any statements necessary to make the statements not misleading. Such an action could hurt our financial condition.
Our issuance of common stock upon exercise of warrants or options may depress the price of our common stock.
As of June 6, 2014, we have 28,947,760 shares of common stock, 7,044,583 shares of common stock issuable upon exchange of the Exchangeable Shares, warrants to purchase 18,732,485, including 9,195,478 Investor Warrants, shares of common stock, and options to purchase 3,240,000 shares of common stock, issued and outstanding. The issuance of shares of common stock upon exercise of outstanding warrants or options could result in substantial dilution to our stockholders, which may have a negative effect on the price of our common stock.
DESCRIPTION OF THE OFFER TO AMEND AND EXERCISE
DelMar Pharmaceuticals, Inc. (the “Company”) is offering to amend, upon the terms and subject to the conditions set forth herein, warrants to purchase an aggregate of 9,195,478 shares of common stock (the “Offer to Amend and Exercise”), including: warrants to purchase 9,195,478 shares of the Company’s common stock issued to investors participating in the Company’s private placement financings closed on January 25, 2013, January 31, 2013, February 8, 2013,February 21, 2013, February 28, 2013, March 1, 2013, and March 6, 2013 (the “ Investor Warrants”). There is no minimum participation requirement with respect to this Offer to Amend and Exercise.
Pursuant to the Offer to Amend and Exercise, the Investor Warrants will be amended (the “ Amended Warrants ” ) to: (i) reduce the exercise price of the Investor Warrants from $0.80 per share to $0.65 per share of common stock in cash, (ii) shorten the exercise period of the Investor Warrants so that they expire concurrently with the expiration of the Offer to Amend and Exercise at 5:00 p.m. (Pacific Time) on July 28, 2014, as may be extended by the Company in its sole discretion (“ Expiration Date”), (iii) delete the price-based anti-dilution provisions contained in the Investor Warrants, (iv) restrict the ability of the holder of shares issuable upon exercise of the Amended Warrants to sell, make any short sale of, loan, grant any option for the purchase of, or otherwise dispose of any of such shares without the prior written consent of the Company for a period twenty (20) days after the Expiration Date (the “Lock-Up Period”); and (v) provide that a holder, acting alone or with others, will agree not to effect any purchases or sales of any securities of the Company in any “short sales” as defined in Rule 200 promulgated under Regulation SHO under the Exchange Act, or any type of direct and indirect stock pledges, forward sale contracts, options, puts, calls, short sales, swaps, “put equivalent positions” (as defined in Rule 16a-1(h) under the Exchange Act) or similar arrangements, or sales or other transactions through non-U.S. broker dealers or foreign regulated brokers through the expiration of the Lock-Up Period. Other than set forth above, the terms of the Investor Warrants will remain unmodified and in full force and effect.
SECTION 1. FORWARD LOOKING STATEMENTS
This Offer to Amend and Exercise contains forward-looking statements. These statements relate to anticipated future events, future results of operations or future financial performance. In some cases, you can identify forward-looking statements by terminology such as “may,” “might,” “will,” “should,” “intends,” “expects,” “plans,” “goals,” “projects,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” or “continue” or the negative of these terms or other comparable terminology. These forward-looking statements are only expectations, are uncertain and involve substantial known and unknown risks, uncertainties and other factors which may cause the Company’s (or its industry’s) actual results, levels of activity or performance to be materially different from any future results, levels of activity or performance expressed or implied by these forward-looking statements. The factors that could cause the Company’s actual results to differ materially from current expectations include, but are not limited to, risks and uncertainties relating to the Company’s ability to develop, market and sell products based on its technology; the expected benefits of the Company’s products and technology; the availability of substantial additional funding for the Company to continue its operations and to conduct research and development, clinical studies and future product commercialization; and, the Company’s business, research, product development, regulatory approval, marketing and distribution plans and strategies. The “Risk Factors” section of this Offer to Amend and Exercise sets forth detailed risks, uncertainties and cautionary statements regarding the Company’s business, the Company’s common stock and the risks of participating in the Offer to Amend and Exercise. You should not place undue reliance on these forward-looking statements, which speak only as of the date that they were made. These cautionary statements should be considered with any written or oral forward-looking statements that the Company may issue in the future. Except as required by applicable law, the Company does not intend to update any of the forward-looking statements to conform these statements to reflect actual results, later events or circumstances or to reflect the occurrence of unanticipated events.
SECTION 2. PURPOSES OF THE OFFER TO AMEND AND EXERCISE AND USE OF PROCEEDS, PLANS OR PROPOSALS
Reduction of Warrant Liability: The Offer to Amend can help the Company reduce the warrant liability recorded by the Company on its financial statements. The warrant liability serves as an impediment to certain goals of the Company, as significant warrant liability on the Company’s balance sheet may make it more difficult for the Company to list its shares of common stock on a national securities exchange. The Investor Warrants contain price-based anti-dilution provisions that provide the holders with protection against future down-round financings. Based on these anti-dilution provisions, the Company is required to record a derivative liability on its balance sheet each fiscal quarter for the Investor Warrants based on the fair value of the Investor Warrants as of the end of such fiscal quarter. The Company’s obligation to continue to record a derivative liability each quarter for a particular Investor Warrant ends when the Investor Warrant is exercised or expires. Various factors are considered in the pricing models the Company used to value the Investor Warrants, including the Company’s current stock price, the remaining life of the Investor Warrants, the volatility of the Company’s stock price, and the risk free interest rate. As a result of the changes in these factors, the warrant derivative liability related solely to the Investor Warrants recorded by the Company was approximately $4,669,731 and $3,511,115 for the periods ended March 31, 2014 and December 31, 2013, respectively. Future changes in these factors will continue to have a significant impact on the computed fair value of the derivative liability for the Investor Warrants. As such, the Company expects future changes in the fair value of the Investor Warrants to continue to vary significantly from quarter to quarter. The Company believes these significant variations make it more difficult for investors to evaluate the Company’s business and operations.
Fund Raising: An additional purpose of the Offer to Amend and Exercise is to raise funds to support the Company’s future operations and capital requirements by encouraging the participating holders to exercise their Investor Warrants by significantly reducing the exercise price and shortening the exercise period. The funds obtained will be used by the Company as working capital and for other general corporate purposes.
Plans or Proposals: The Company intends to cancel the Investor Warrants upon the exercise of the Investor Warrants by the holders thereof. Investor Warrants that are not so exercised pursuant to this Offer to Amend and Exercise will remain outstanding pursuant to their original terms.
No plans or proposals described in this Offer to Amend and Exercise or in any materials sent to the holders of the Investor Warrants in connection with this Offer to Amend and Exercise relate to or would result in the conditions or transactions described in Regulation M-A, Item 1006(c)(1) through (10), except as follows:
Any holder of Investor Warrants who elects to exercise his, her or its Investor Warrants will acquire additional shares of common stock of the Company as a result of such exercise. As of June 6, 2014, the Company had 28,947,760 shares of common stock outstanding. The Investor Warrants are exercisable for an aggregate of 9,195,478 shares of common stock. Assuming all Investor Warrants are exercised, the Company’s outstanding shares of common stock would increase to 38,143,238 shares, with the shares issued upon exercise of the Investor Warrants representing 24% of the then outstanding shares of common stock.
SECTION 3. ELIGIBILE INVESTOR WARRANTS
The following Investor Warrants are subject to the Offer to Amend and Exercise:
Investor Warrants: Outstanding warrants to purchase 9,195,478 shares of the Company’s common stock issued to investors participating in the Company’s private placement financings closed on January 25, 2013, January 31, 2013 , February 8, 2013, February 21, 2013, February 28, 2013, March 1, 2013, and March 6, 2013, as amended.
SECTION 4. EXPIRATION DATE
The Offer to Amend and Exercise will be open through 5:00 p.m., Pacific Time on July 28 , 2014, as may be extended by the Company in its sole discretion.
SECTION 5. TERMS OF AMENDED WARRANTS
Pursuant to the Offer to Amend and Exercise, the Investor Warrants will be amended as described below:
New Exercise Price: The exercise price of the Investor Warrants will be reduced from $0.80 per share to $0.65 per share.
New Termination Date: The termination date of the Investor Warrants is being shortened to run concurrently with the Expiration Date.
Lock-Up Period: The Amended Warrants will contain a lock-up provision that provides that the holder will not sell, make any short sale of, loan, grant any option for the purchase of, or otherwise dispose of any of the shares issuable upon exercise of the Amended Warrants without the prior written consent of the Company for a period of twenty (20) days after the Expiration Date. In addition, the Company may impose stop-transfer restrictions to enforce these restrictions.
No Cashless Exercise: The Amended Warrants must be exercised for cash, and any cashless exercise provisions in the Investor Warrants will be inapplicable to the Offer to Amend and Exercise. The shares of common stock issuable upon the exercise of the Amended Warrants will be issued to the holder promptly after the holder’s exercise of the Amended Warrants.
Anti-Dilution: The price-based anti-dilution provisions contained in the Investor Warrants will be deleted. Any price-based anti-dilution provisions in the Investor Warrants will be inapplicable to the Offer to Amend and Exercise.
Market Restrictions: A holder will agree not to effect any purchases or sales of any securities of the Company, acting alone or with others, in any “short sales” as defined in Rule 200 promulgated under Regulation SHO under the Exchange Act, or any type of direct and indirect stock pledges, forward sale contracts, options, puts, calls, short sales, swaps, “put equivalent positions” (as defined in Rule 16a-1(h) under the Exchange Act) or similar arrangements, or sales or other transactions through non-U.S. broker dealers or foreign regulated brokers through the expiration of the Lock-Up Period.
Other Terms: Except as set forth above all other terms of the Amended Warrants will be the same as the terms of the Investor Warrants. See the form of Amended Warrant attached hereto as Exhibits (a)(1)(E) to the Schedule TO.
SECTION 6. CONDITIONS TO THE OFFER TO AMEND AND EXERCISE
The Offer to Amend and Exercise is subject to certain conditions, as described herein:
|
(i)
|
As part of the Election to Participate and Exercise Warrant, the holders of the Investor Warrants must complete an Accredited Investor Questionnaire. The holders of the Investor Warrants previously represented to the Company that they were “accredited investors” in connection with the transactions in which such holders acquired the Investor Warrants. The holders must complete this questionnaire even if they are no longer “accredited investors”. The Company has included with this Offer to Amend and Exercise an exhibit titled “Supplemental Company Information” that contains additional information that holders of Investor Warrants who are no longer “accredited investors,” if any, should consider before making an investment decision.
|
|
(ii)
|
In addition, we are not making this Offer to Amend and Exercise to, nor will we accept any Election to Participate and Exercise Warrant from or on behalf of, Investor Warrant holders in any jurisdiction in which the Offer to Amend and Exercise or the exercise of the Amended Warrants would not be in compliance with the laws of such jurisdiction.
|
You may not elect to amend but not exercise your Investor Warrants. Participation in this Offer to Amend and Exercise requires both amendment of your Investor Warrants and your exercise of the Amended Warrants, which will happen simultaneously should you choose to participate.
Investor Warrants of holders that elect not to participate and exercise will remain outstanding pursuant to their original terms.
SECTION 7. EXTENSION OF OFFER TO AMEND AND EXERCISE PERIOD; TERMINATION; AMENDMENTS
The Company expressly reserves the right, in its sole discretion and at any time or from time to time, to extend the Expiration Date.
There can be no assurance, however, that the Company will exercise its right to extend the Offer to Amend and Exercise. Amendments to the Offer to Amend and Exercise will be made by written notice thereof to the holders of the Investor Warrants. Material changes to information previously provided to holders of the Investor Warrants in this Offer to Amend and Exercise or in documents furnished subsequent thereto will be disseminated to holders of Investor Warrants. Also, should the Company, pursuant to the terms and conditions of the Offer to Amend and Exercise, materially amend the Offer to Amend and Exercise, the Company will ensure that the Offer to Amend and Exercise remains open long enough to comply with U.S. federal securities laws.
If the Company materially changes the terms of the Offer to Amend and Exercise or the information concerning the Offer to Amend and Exercise, or it waives a material condition of the Offer to Amend and Exercise, the Company will extend the Offer to Amend and Exercise to the extent required under applicable law. The minimum period during which an offer must remain open following any material change in the terms of the Offer to Amend and Exercise or information concerning the Offer to Amend and Exercise (other than a change in price, change in dealer’s soliciting fee or change in percentage of securities sought all of which require up to ten (10) additional business days) will depend on the facts and circumstances, including the relative materiality of such terms or information.
SECTION 8. PROCEDURE FOR PARTICIPATING IN OFFER TO AMEND AND EXERCISE AND EXERCISING AMENDED WARRANTS
To participate in the Offer to Amend and Exercise accept and exercise an Amended Warrant and receive the number of shares of Company common stock issuable therefor, you must deliver to the Company before the Expiration Date all of the following: (i) a signed copy of the Election to Participate and Exercise Warrant, (ii) a signed copy of an Accredited Investor Questionnaire, (iii) the original copy of your Investor Warrant (or an Affidavit of Lost Warrant) for cancellation, and (iv) cash in the amount equal to $0.65 per share multiplied by the number of shares of common stock the holder elects to purchase (collectively, the “Acceptance and Exercise Documents”). The cash exercise price may be tendered in the form of a check payable to Signature Bank as Escrow Agent for DelMar Pharmaceuticals, Inc. or by wire transfer to the Company’s escrow account at Signature Bank as set forth in the Election to Participate and Exercise Warrant. The signed copy of the Election to Participate and Exercise Warrant, the signed copy of the Accredited Investor Questionnaire, and the original copy of the Investor Warrant (or an Affidavit of Lost Warrant) for cancellation must be properly delivered, before the Expiration Date to: DelMar Pharmaceuticals, Inc., Suite 720 -- 999 West Broadway, Vancouver, British Columbia CANADA V5Z 1K5, Attn: Corporate Secretary, telephone number (604) 629-5989.
SECTION 9. MANNER OF ACCEPTANCE OF PAYMENT AND ISSUANCE OF SHARES
If you properly tender (and do not validly withdraw) your Investor Warrants and the other Acceptance and Exercise Documents on or prior to 5:00 p.m., Pacific Time on July 28 , 2014, the Expiration Date of the Offer to Amend and Exercise (or such later date and time if we extend the Offer to Amend and Exercise), promptly following the Expiration Date, we intend to notify our depositary institution and our transfer agent of our acceptance of your payment of the exercise price and your other Acceptance and Exercise Documents and issue and deliver to you the number of shares of Company common stock issuable under the Amended Warrant . See Section 8 “Procedure for Participating in Offer to Amend and Exercise and Exercising Amended Warrants” below.
SECTION 10 WITHDRAWAL RIGHTS
If you change your mind and do not want to participate in the Offer to Amend and Exercise, you may submit the Notice of Withdrawal to us. However, to be effective, the Notice of Withdrawal must be properly completed and must be returned, before 5:00 p.m. Pacific Time on July 28 , the Expiration Date of the Offer to Amend and Exercise (or such later date and time if we extend the Offer to Amend Exercise), the Expiration Date to: DelMar Pharmaceuticals, Inc., Suite 720 -- 999 West Broadway, Vancouver, British Columbia CANADA V5Z 1K5, Attn: Corporate Secretary, telephone number (604) 629-5989. Following the Expiration Date, you cannot withdraw your Election to Participate and Exercise Warrant. However, if we have not accepted your tendered Investor Warrants and other Acceptance and Exercise Documents by July 28, 2014, which is atleast twenty business day s from the commencement of the Offer to Amend and Exercise, you may change your mind and submit a Notice of Withdrawal to us after July 28, 2014.
If you properly withdraw in a timely manner as set forth above, we will promptly: (i) cancel your signed copy of the Election to Participate and Exercise Warrant, (ii) return the original copy of your Investor Warrant (which will remain unmodified and in full force and effect), or issue you a new Investor Warrant if you submitted an Affidavit of Lost Warrant, and (iii) provide you with a check equal to the amount of cash you paid upon exercise of the Amended Warrant.
SECTION 11. REGISTRATION OF WARRANT SHARES
The Investor Warrants and the shares of common stock issuable upon exercise of the Amended Warrants are “restricted securities” and may not be sold by the holder absent a registration statement covering the resale of the shares or an exemption from the registration requirement. We have previously filed a Registration Statement on Form S-1 (File No. 333-182101) to register the resale of the shares of common stock underlying the Investor Warrants under the Securities Act, and amending the Investor Warrants through the Offer to Amend and Exercise will not affect the registration for holders named as selling shareholders in the Registration Statement. Promptly following the Expiration Date, we intend to file a prospectus supplement to the prospectus included in the Registration Statement to reflect the substantive changes from the information currently set forth in such prospectus as a result of the Offer to Amend and Exercise. Thereafter, the holders of shares of common stock issuable upon exercise of the Amended Warrants who are listed as selling stockholders in the Registration Statement may sell their shares of common stock in accordance with the resale restrictions set forth in the “Plan of Distribution” section of the Prospectus in the Registration Statement. Each holder of Investor Warrants should read the applicable Prospectus carefully before deciding whether to participate in the Offer to Amend and Exercise. In addition, any holder (including any transferees or acquirers) of an Investor Warrant or Amended Warrant who is not listed as a selling stockholder in the Prospectus cannot resell such holder’s shares in reliance on the Prospectus, unless and until the Company files a post-effective amendment to the Registration Statement to include such holder as a selling stockholder. Absent the filing of the post-effective amendment to the Registration Statement, the holder (including any transferees or acquirers) will be required to qualify for an exemption from the registration requirements, which may require a holding period of at least six months.
SECTION 12. TRADING MARKET AND PRICE RANGE OF COMMON STOCK
There is no established trading market for the Investor Warrants or the Amended Warrants.
The Company’s common stock is quoted on the Over-the-Counter Bulletin Board, or OTCBB, under the symbol “DMPI.”
There was no reported trading in our common stock prior to January 25, 2013. Since January 25, 2013, there has been limited trading in our common stock. The following table sets forth the range of high and low bid prices of our common stock as reported and summarized on the OTCQB for the periods indicated. These prices are based on inter-dealer bid and asked prices, without markup, markdown, commissions, or adjustments and may not represent actual transactions.
|
Calendar Quarter
|
|
High Bid
|
|
|
Low Bid
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trades in our common stock may be subject to Rule 15g-9 of the Exchange Act, which imposes requirements on broker/dealers who sell securities subject to the rule to persons other than established customers and accredited investors. For transactions covered by the rule, broker/dealers must make a special suitability determination for purchasers of the securities and receive the purchaser’s written agreement to the transaction before the sale.
The SEC also has rules that regulate broker/dealer practices in connection with transactions in “penny stocks.” Penny stocks generally are equity securities with a price of less than $5.00 (other than securities listed on certain national exchanges, provided that the current price and volume information with respect to transactions in that security is provided by the applicable exchange or system). The penny stock rules require a broker/dealer, before effecting a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document prepared by the SEC that provides information about penny stocks and the nature and level of risks in the penny stock market. The broker/dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker/dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker/dealer and salesperson compensation information, must be given to the customer orally or in writing before effecting the transaction, and must be given to the customer in writing before or with the customer’s confirmation. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for shares of our common stock. As a result of these rules, investors may find it difficult to sell their shares.
SECTION 13. SOURCE AND AMOUNT OF FUNDS
Because this transaction is solely an offer to holders to amend their outstanding Investor Warrants, there are no funds or other consideration being paid to participants. The Company will use its existing working capital to pay the fees and expenses associated with this Offer to Amend and Exercise.
SECTION 14. TRANSACTIONS AND AGREEMENTS CONCERNING INVESTOR WARRANTS
None of our directors or executive officers participated in any transaction involving the Investor Warrants during the past 60 days.
SECTION 15. INFORMATION REGARDING THE COMPANY
The following summary highlights selected information regarding the Company. Because it is a summary, it does not contain all of the information you should consider before making a decision to participate in the Offer to Amend and Exercise or exercise your Amended Warrant. Before making an investment decision, you should read the entire Offer to Amend and Exercise carefully, including the “Risk Factors” section above.
Overview
DelMar Pharmaceuticals, Inc. (the “Company”) is a Nevada corporation formed on June 24, 2009 under the name Berry Only Inc. (“Berry”). Prior to the Reverse Acquisition (discussed below), Berry did not have any significant assets or operations. On January21, 2013, the Company changed its name to DelMar Pharmaceuticals, Inc.
DelMar Pharmaceuticals, Inc. is the parent company of Del Mar Pharmaceuticals (BC) Ltd. (“DelMar (BC)”), a British Columbia, Canada corporation incorporated on April 6, 2010, which is a clinical and commercial stage drug development company with a focus on the treatment of cancer. We are conducting clinical trials in the United States with our lead product, VAL-083, as a potential new treatment for GBM, the most common and aggressive form of brain cancer. We have also acquired certain exclusive commercial rights to VAL-083 in China where it is approved as a chemotherapy for the treatment of chronic myelogenous leukemia (“CML”) and lung cancer. We plan to seek marketing partnerships in China in order to generate royalty revenue.
Our executive offices are located at Suite 720-999 West Broadway, Vancouver, British Columbia, Canada V5Z 1K5. Our clinical operations are managed at Suite R, 3475 Edison Way, Menlo Park, California, 94025. Our website is located at www.delmarpharma.com, and our telephone number is 604-629-5989.
On January 25, 2013 (the “Closing Date”), the Company entered into and closed an exchange agreement (the “Exchange Agreement”), with DelMar (BC), 0959454 B.C. Ltd., a British Columbia corporation and a wholly-owned subsidiary of the Company (“Callco”), 0959456 B.C. Ltd., a British Columbia corporation and a wholly-owned subsidiary of the Company (“Exchangeco”), and securityholders of DelMar (BC). Pursuant to the Exchange Agreement, (i) the Company issued 4,340,417 shares of common stock (the “Parent Shares”) to the shareholders of DelMar (BC) who are United States residents (the “U.S. Holders”) in exchange for the transfer to Exchangeco of all 4,340,417 outstanding common shares of DelMar (BC) held by the U.S. Holders, (ii) the shareholders of DelMar (BC) who are Canadian residents (the “Canadian Holders”) received, in exchange for the transfer to Exchangeco of all 8,729,583 outstanding common shares of DelMar (BC) held by the Canadian Holders, 8,729,583 exchangeable shares (the “Exchangeable Shares”) of Exchangeco, and (iii) outstanding warrants to purchase 3,360,000 common shares of DelMar (BC) and outstanding options to purchase 1,020,000 common shares of DelMar (BC) were deemed to be amended such that, rather than entitling the holder to acquire common shares of DelMar (BC), such options and warrants (as amended, the “Exchange Agreement Warrants”) will entitle the holders to acquire shares of common stock of the Company. The Canadian Holders will be entitled to require Exchangeco to redeem (or, at the option of the Company or Callco, to have the Company or Callco purchase) the Exchangeable Shares, and upon such redemption or purchase to receive an equal number of shares of common stock of the Company.
Effective on the Closing Date, pursuant to the Exchange Agreement, DelMar (BC) became (indirectly through Exchangeco) a wholly-owned subsidiary of the Company. The acquisition of DelMar (BC) is treated as a reverse acquisition, and the business of DelMar (BC) became the business of the Company. At the time of the Reverse Acquisition, Berry was not engaged in any active business.
Our mission is to benefit patients and create shareholder value by rapidly developing and commercializing anti-cancer therapies in orphan cancer indications where patients have failed or are unlikely to respond to modern therapy. Our lead product candidate, VAL-083, represents a “first-in-class” small-molecule chemotherapeutic, which means that the molecular structure of VAL-083 is not an analogue or derivative of other small molecule chemotherapeutics approved for the treatment of cancer. VAL-083 has been assessed in multiple clinical studies sponsored by the National Cancer Institute (“NCI”) in the United States as a treatment against various cancers including lung, brain, cervical, ovarian tumors and leukemia. Published pre-clinical and clinical data suggest that VAL-083 may be active against a range of tumor types. VAL-083 is approved as a cancer chemotherapeutic in China for the treatment of CML and lung cancer. VAL-083 has not been approved for any indication outside of China.
Upon obtaining regulatory approval, we intend to commercialize VAL-083 for the treatment of orphan and other cancer indications where patients have failed other therapies or have limited medical options. Orphan diseases are defined in the United States under the Rare Disease Act of 2002 as “any disease or condition that affects less than 200,000 persons in the United States”. The Orphan Drug Act of 1983 is a federal law that provides financial and other incentives including a period of market exclusivity to encourage the development of new treatments for orphan diseases. In February 2012, we announced that VAL-083 has been granted protection under the Orphan Drug Act by the United States Food and Drug Administration (“FDA”) for the treatment of glioma, including GBM. In January 2013, the European Medicines Agency (“EMA”) also granted orphan drug protection to VAL-083 for the treatment of glioma.
We research the mechanism of action of our product candidate to determine the clinical indications best suited for therapy and work rapidly advance it into human clinical trials and toward commercialization. With this aim, in October 2011 we initiated clinical trials with VAL-083 as a potential new treatment for GBM, the most common and aggressive form of brain cancer. We have presented interim data from our clinical trial at peer reviewed scientific meetings demonstrating that VAL-083 can shrink or halt the growth of tumors in some brain cancer patients who have failed other approved treatments. Currently, there is no approved therapy for these patients.
In addition to our clinical development activities in the United States, we have obtained exclusive commercial rights to VAL-083 in China. In October 2012, we announced that we had entered into a collaboration agreement with the only manufacturer presently licensed by the China Food and Drug Administration (“CFDA”) to produce the product for the China market. This agreement provides us with exclusive commercial rights which potentially position us to generate near-term revenue through product sales or royalties for its approved indications in China while we seek global approval in new indications. We anticipate that we may be able to begin generating revenue from such sales or royalties commencing in 2014.
VAL-083 was originally discovered in the 1960’s. We have filed a broad portfolio of new patent applications to protect our intellectual property. Our patent applications claim compositions and methods related to the use of VAL-083 and related compounds as well as methods of synthesis and quality controls for the manufacturing process of VAL-083. In July 2013, our first patent was granted by the United States Patent and Trademark Office. The patent expiration date is August 17, 2031. In addition, VAL-083 has been granted protection under the Orphan Drug Act by the FDA and the EMA. We believe that our portfolio of intellectual property rights provides a strong and defensible market position for the commercialization of VAL-083 and other anti-cancer products.
We also believe the experience of our clinical development team will position us to acquire or license additional product candidates to establish a pipeline of product opportunities. We have secured three grants from the National Research Council of Canada, which have provided financial contributions of over Cdn $130,000 to date. We believe we have the potential to create significant value by building and maintaining a sustainable business through the commercialization of VAL-083 across a variety of cancer indications on a world-wide basis.
The Technology
Our drug discovery research focuses on identifying well-validated clinical and commercial-stage compounds and establishing a scientific rationale for development in modern orphan drug indications. Through our relationship with Valent Technologies, LLC (“Valent”), a company owned by Dr. Dennis Brown, our Chief Scientific Officer, we are able to utilize Valent’s proprietary ChemEstate™ bioinformatics tools which are used to screen and identify potential candidates. Promising candidates are further researched through our network of consultants and contract research organizations. This approach allows us to rapidly identify and advance potential drug candidates without significant investment in “wet lab” infrastructure. Based on this strategy, we acquired initial VAL-083 intellectual property and prototype drug product from Valent and have identified multiple additional drug candidates that we may have the opportunity to license or acquire in the future.
VAL-083
VAL-083 is a novel “first in class” small-molecule therapeutic agent that we are developing as a new cancer chemotherapy.
VAL-083 has been assessed in multiple National Cancer Institute (“NCI”)-sponsored clinical studies in various cancers including lung, brain, cervical, ovarian tumors and leukemia. Published pre-clinical and clinical data from the late 1970s and 1980s suggest that VAL-083 may be active against a range of tumor types; however, further research was not pursued in the United States due to an increased focus by the NCI on targeted biologic therapies during the era. VAL-083 is approved as a cancer chemotherapeutic in China for the treatment of CML and lung cancer.
The mechanism of action of VAL-083 is understood to be a bi-functional alkylating agent. Alkylating agents are a commonly used class of chemotherapy drugs. They work by binding to DNA and interfering with normal processes within the cancer cell, which prevents the cell from making the proteins needed to grow and survive. After exposure to alkylating agents, the cancer cell becomes dysfunctional and dies. There are a number of alkylating agents on the market that are used by physicians to treat different types of cancer.
Based on published research, the functional groups associated with the mechanism of action of VAL-083 are understood to be functionally different from commonly used alkylating agents, including Temodar®, which is commonly used a front-line chemotherapy against GBM. VAL-083 has previously demonstrated activity in cell-lines that are resistant to other types of chemotherapy. No evidence of cross-resistance has been reported in published clinical studies. Based on the presumed alkylating functionality of VAL-083, published literature suggests that DNA repair mechanisms associated with the leading brain cancer therapies, including Temodar ® and nitrosourea resistance, may not confer resistance to VAL-083. Therefore, we believe that VAL-083 may be effective in treating tumors that have failed or become resistant to other chemotherapies.
We have presented new research at peer-reviewed scientific meetings demonstrating that VAL-083 is active in some patients, patient-derived tumor cell lines and cancer stem cells that are resistant to other chemotherapies. Of particular importance is resistance to Temodar ® due to activity of the repair enzyme known as MGMT, which results in resistance to front-line therapy in many GBM patients. At AACR in 2012, we presented data demonstrating that VAL-083 is active independent of MGMT resistance in laboratory studies.
VAL-083 readily crosses the blood brain barrier where it maintains a long half-life in comparison to the plasma. Published preclinical and clinical research demonstrates that VAL-083 is selective for brain tumor tissue.
VAL-083 has been assessed in multiple studies as chemotherapy in the treatment of newly diagnosed and recurrent brain tumors and other cancers. In general, tumor regression in brain cancer was achieved following therapy in greater than 40% of patients treated and stabilization was achieved in an additional 20% - 30%. In published clinical studies, VAL-083 has previously been shown to have a statistically significant impact on median survival in high grade glioma brain tumors when combined with radiation vs. radiation alone.
A summary of published data adapted from separate sources comparing the efficacy of VAL-083 and other therapiesin the treatment of glioblastoma multiforme (GBM).
The main dose-limiting toxicity (“DLT”) related to the administration of VAL-083 in previous NCI-sponsored clinical studies was myelosuppression. Myelosuppression is the decrease in cells responsible for providing immunity, carrying oxygen, and those responsible for normal blood clotting. Myelosuppression is a common side effect of chemotherapy. There is no evidence of lung, liver or kidney toxicity even with prolonged treatment by VAL-083. Commercial data from the Chinese market where the drug has been approved for more than 15 years supports the safety findings of the NCI studies.
We note that the DLT of VAL-083 was established prior to the development of medicines now available to manage myelosuppression. Various types of medications and other forms of therapy are now available for management of myelosuppressive side effects. We believe this offers the potential of increasing the dose of VAL-083 in the modern patient population thereby providing a potential opportunity to improve the drugs already established efficacy profile.
VAL-083 Clinical Development in GBM
Based on historical data and our own research, we filed an investigational new drug (“IND”) application with the FDA and initiated human clinical trials with VAL-083 as a potential treatment for GBM in 2011.
Our clinical trial is a Phase I/II an open-label, single arm dose-escalation study designed to evaluate the safety, tolerability, pharmacokinetics and anti-cancer activity of VAL-083 in patients with GBM. To be eligible for our clinical trial, patients must have been previously treated for GBM with surgery and/or radiation, if appropriate, and must have failed both Bevacizumab (Avastin ®) and temozolomide (Temodar ®), unless either or both are contra-indicated. .
Response to treatment with VAL-083 is measured prior to each treatment cycle. An initial phase of the study involves dose escalation cohorts until a maximum tolerated dose (“MTD”) is established in the context of modern care. The goal of our Phase I/II clinical trial is to determine a modernized dosing regimen for advancement into a registration directed clinical trial.
In February 2012, we announced that VAL-083 was granted protection under the Orphan Drug Act by the FDA for the treatment of glioma. In January 2013, we announced that the European Union had also granted orphan drug protection to VAL-083. Orphan drugs generally follow the same regulatory development path as any other pharmaceutical product. However, incentives such as scientific advice and reduction or waiver of registration fees and access to specialized grant funding may be available to support and accelerate development of orphan drug candidates. In addition, DelMar Pharma may sell VAL-083 as a treatment for glioma without competition for seven years in the US and for ten years in the EU following market approval, in respect of a medicinal product containing a similar active substance for the same indication.
Based on historical development of other products in GBM, we believe that we may be able to obtain FDA approval to commercialize VAL-083 to treat patients who have failed other therapies from an open-label Phase II registration-directed clinical, which will save significant costs of a large Phase III clinical trial. We also believe that the FDA may grant fast-track, accelerated approval and/or priority review status to VAL-083, which will enable us to begin filing for commercial approval during the clinical trial process. Fast Track, Accelerated Approval and Priority Review are approaches established by the FDA that are intended to make therapeutically important drugs available at an earlier time. (See “Government Regulation and Product Approval”.)
We are conducting the study under the direction of Dr. Howard Burris at the Sarah Cannon Research Institute in Nashville, Tennessee with a second center in Sarasota, Florida. In July 2013, the Company announced the opening of its third clinical trial site at the Brain Tumor Center at University of California, San Francisco (“UCSF”).
We have presented interim data from our clinical trial at peer-reviewed scientific meetings including the Society for NeuroOncology annual meeting (“SNO” – November, 2012), the American Association of Cancer Research (“AACR” – April 2013), the American Society for Clinical Oncology (“ASCO” – June 2013), the World Federation of Neuro‐Oncology (“WFNO” – November, 2013), AACR in April 2014, and ASCO in May 2014. In summary, our interim clinical data supports that:
|
●
|
To date, one of two GBM patients in cohort 6 (30 mg/m2) exhibited stable disease after one cycle of treatment. Outcomes and analysis of cohorts 6 and 7 are ongoing;
|
|
●
|
In earlier cohorts, DelMar reported that two patients exhibited a response (stable disease or partial response) with a maximum response of 28 cycles (84 weeks) and improved clinical signs prior to discontinuing due to adverse events unrelated to study;
|
|
●
|
No drug-related serious adverse events have been detected, and maximum tolerated dose (“MTD”) has not been reached at doses up to 30 mg/m2. Enrollment and evaluation of Cohort 7 (40mg/m2) is ongoing;
|
|
●
|
DelMar has also presented data demonstrating that the cytotoxic activity of VAL-083 is independent of MGMT, the enzyme believed to cause resistance to the current front-line therapy in the treatment of GBM; and
|
|
●
|
Pharmacokinetics are linear and consistent with previous published data suggesting that concentrations of VAL-083 being obtained are effective against glioma cell lines in vitro.
|
These data support the further development of VAL-083. We are continuing with the dose escalation portion of our clinical trial and anticipate achieving the maximum tolerated dose during 2014.
In August 2013, the Company received a notice of allowance from the FDA enabling the Company to implement a more rapid dose-escalation scheme in our GBM study. The revised dosing regimen was allowed by the FDA following an extensive safety review of patients treated to date. In comparison to the original dose-escalation scheme, the revised plan will enable the trial to reach higher doses and complete the dose-escalation portion of the clinical trial more quickly by skipping two interim doses.
A summary of our original and revised dose escalation scheme including doses completed to date is as follows:
|
Dose Escalation Scheme (mg/m2)
|
Patients Treated
|
Status
|
|
Original
|
Revised
|
|
|
|
|
Completed – No Dose Limiting Toxicity, or “DLT”
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
*Cohorts 2 and 3 were expanded to allow for patient demand and to gather additional data on CNS metastases patients.
|
If the MTD is not reached in cohort 7, DelMar would be prepared to file a protocol amendment with the FDA to allow dosing beyond 40mg/m2. During the remainder of 2014 we plan to continue our clinical trials with VAL-083 as a potential treatment for GBM patients who have failed other therapies. Currently, there is no approved therapy for these patients. The goal of the current trial is to establish a modernized dosing regimen for advancement into registration directed trials in the United States as a potential new therapy for the treatment of refractory GBM.
As part of our ASCO presentation on June 1, 2013, we also announced that we plan to split our current clinical trial protocol into two separate studies: one focusing solely on refractory GBM and the other focusing on secondary brain cancers caused by other tumors that have spread to the brain. Due to prior chemotherapy and radiation therapy, patients with secondary brain tumors are likely more prone to myelosuppression and may have a different toxicity and MTD than patients with GBM. We believe the strategy of splitting the trial into two separate studies will enable us to focus on accelerating the development of VAL-083 as a potential new treatment for glioblastoma while appropriately exploring the potential of the drug to treat patients with solid tumors that have spread to the brain.
We anticipate presenting additional data at upcoming scientific meetings during 2014.
The current study is being conducted under an IND application with the FDA. It involves a dose-escalation phase (Phase I) and an efficacy phase (Phase II). Phase I of the study will continue to enroll patients until a MTD is achieved. Based on historical data, we anticipate that Phase I will involve up to 30 patients. In the Phase II portion of the current study, an additional 14 GBM patients can be enrolled at the MTD or a lower dose recommended by the principal investigator. Details of the study, including enrollment estimates, are available at http://www.clinicaltrials.gov/ct2/show/NCT01478178?term=VAL-083&rank=1). We plan to develop a separate protocol for the continued exploration of VAL-083 in patients with secondary brain cancer caused by solid tumor spreading to the brain.
While our data with VAL-083 to date are interim in nature, we believe the results to date demonstrate a strong potential for successful development of VAL-083 as a chemotherapy for the treatment of GBM. We plan to continuing working with our clinical investigators to determining an optimal dosing regimen for future registration trials.
VAL-083 in Leukemia and Hematologic Cancers
CML, also known as chronic myeloid leukemia, is a cancer of the white blood cells. The incidence of CML in the United States is approximately two per 100,000 population.
CML is characterized by three progressive phases: chronic, aggressive and blast, each corresponding with poorer prognosis. Approximately 85% of patients with CML are in the chronic phase at the time of diagnosis. Chronic phase patients are usually asymptomatic or have only mild symptoms such as fatigue or no symptoms at all. The duration of chronic phase is variable and depends on how early the disease was diagnosed as well as type of treatment. Without treatment, CML progresses to an accelerated phase and eventually to blast crisis. Blast crisis is the final phase in the evolution of CML and behaves like an acute leukemia with rapid progression and short expected survival.
VAL-083 has shown promise in CML in multiple pre-clinical and clinical studies. The NCI studied VAL-083 extensively in laboratory and animal models of hematological malignancies (blood cancers). VAL-083 has been approved for the treatment of CML in China. While VAL-083 maintains labeling for CML in China, use of the drug in the modern era has been limited by a preference for targeted therapies such as tyrosine kinase inhibitors (“TKIs”).
TKIs have become the standard of care for CML and non-small cell lung cancer (“NSCLC”). TKI therapy has resulted in vastly improved outcomes; however, patients often develop resistance to TKI therapy. Recent evidence proposes unique mechanisms of resistance in patients of East Asian descent who experience significantly inferior responses to TKIs, including imatinib (Gleevec ®) in CML and erlotinib (Tarceva ®) in lung cancer.
We believe that data from NCI-sponsored studies and commercial evidence from the Chinese market support substantive clinical benefit of VAL-083 in CML. We also believe that the unique mechanism of action of VAL-083, in combination with newly developed data positions the drug as a valuable therapy for patients who have failed other treatments, including TKIs. This represents a significant clinical and commercial opportunity for large subsets of patient populations in the existing-approved China market as well as for global development in CML.
Based on these beliefs, we have acquired certain commercial rights to VAL-083 in China where it is approved for the treatment of CML and Lung Cancer. We have also developed new non-clinical data demonstrating that VAL-083 is active against TKI-resistant CML. We have begun to establish a network of leading oncologists to develop new clinical and non-clinical data which will demonstrate the clinical utility of VAL-083 in CML patients who are resistant to TKIs. We believe this strategy will result in sales growth for VAL-083 in China and generate near-term revenue for our company through sales and marketing partnerships as well as position VAL-083 for global development in CML.
In addition, we plan to investigate VAL-083 as a potential treatment for other types of blood cancer. Acute Myeloid Leukemia (“AML”) and Acute Lymphoblastic Leukemia (“ALL”) are of particular interest based on published data and lack of effective therapeutic options. We have initiated preliminary discussions with leading cancer centers regarding the development of a clinical strategy for the development of VAL-083 in other types of blood cancer.
VAL-083 in Lung Cancer
Lung cancer is characterized as small cell and non-small cell lung cancer (“NSLSC”). NSCLC is the most common type of lung cancer.
There are three common forms of NSCLC: adenocarcinomas are often found in an outer area of the lung; squamous cell carcinomas are usually found in the center of the lung next to an air tube (bronchus); and large cell carcinomas, which can occur in any part of the lung and tend to grow and spread faster than adenocarcinoma.
Smoking is the most important risk factor in the development of lung cancer. According to the World Cancer Report (2008), 21% of cancer deaths are related to smoking, especially lung cancer. Additionally, high levels of air pollution have been implicated as significant causes of lung cancer. Incidence of lung cancer in the United States is approximately 59 per 100,000 with the majority (52:100,000) being NSCLC.
According to The Nationwide Nutrition and Health Survey (2002), China has the world’s largest smoking population, with a smoking rate of 24.0% on average (50.2% for men and 2.8% for women), and a total number of 350 million smokers. The World Health Organization reports that the incidence of lung cancer in China is 34 per 100,000 population. However, some estimates are much higher exceeding 120 per 100,000 population for males aged 55-60 in urban areas.
According to a survey conducted by the Chinese Ministry of Health and the Ministry of Science and Technology, smoking, poor diet, water pollution and environmental problems have caused the nation's cancer death rate to rise 80 percent in the past 30 years and cancer is now accountable for 25 percent of all urban deaths and 21 percent of all rural deaths. Based on these trends, the World Health Organization projects that the incidence of lung cancer in China is expected to exceed one million (1,000,000) new cases per year by 2025.
Similar to CML treatment, TKIs are standard front-line therapy in certain types of NSCLC; however resistance to TKI therapy is common in lung cancer patients. It has also been reported that cigarette smoke may directly induce resistance to TKIs. This factor could further exacerbate resistance to modern targeted therapies in populations such as China where smoking is highly prevalent. In addition, the same East-Asian specific resistance linked to TKI-resistance in CML has been shown to correlate with TKI-resistance in NSLSC.
The activity of VAL-083 against lung cancer was studied extensively by the NCI. VAL-083 demonstrated activity against NSCLC in laboratory and animal studies. VAL-083 was also investigated in a number of clinical trials in the United States and Europe during the 1970s both as a stand-alone therapy and in combination with other chemotherapeutic regimens. VAL-083 has been approved for the treatment of lung cancer in China; however, we believe that the use of the drug in the modern era has been limited by a preference for targeted therapies such as TKIs.
We believe VAL-083’s unique bi-functional alkylating mechanism of action could make it a valuable drug of choice in NSCLC patients who are or become resistant to TKI therapy. In addition, VAL-083 readily crosses the blood brain barrier suggesting that it may be possible for VAL-083 to treat patients whose lung cancer has spread to the brain.
Based on these beliefs, we have acquired certain commercial rights to VAL-083 in China where it is approved for the treatment of lung cancer. We plan to work with leading oncologists to develop new clinical and non-clinical data which will demonstrate the clinical utility of VAL-083 in NSCLC patients who are resistant to TKIs. We believe this strategy will result in sales growth for VAL-083 in China and generate near-term revenue for our company through sales and marketing partnerships as well as position VAL-083 for global development in lung cancer. In April 2014 at AACR we announced results of pre-clinical study designed to evaluate the activity of VAL-083 in in vivo models of drug-resistant NSCLC in comparison to cisplatin.
In an established murine xenograft model of NSCLC, the activity of VAL-083 was compared to standard platinum-based therapy with cisplatin against human NSCLC cell lines A549 (TKI-sensitive) and H1975 (TKI-resistant). In the study, VAL-083 demonstrated superior efficacy and safety in the treatment of TKI-susceptible (A549) tumors and in TKI-resistant (H1975) tumors.
|
●
|
Treatment of TKI-sensitive (A549) NSCLC with 3 mg/kg of VAL-083 resulted in tumor growth delay of 26 days compared to untreated controls. Cisplatin (5 mg/kg) resulted in tumor growth delay of just four days. In addition, mean tumor volume on day 68 was significantly reduced in animals treated with 3 mg/kg VAL-083 (p=0.001) compared to untreated control.
|
|
●
|
Treatment of TKI-resistant (H1975) NSCLC with 4 mg/kg of VAL-083 resulted in a statistically significant reduction in tumor volume (p = 0.01) versus untreated control after 27 days. In the same model, treatment with 5 mg/kg of cisplatin failed to achieve statistically significant reduction in tumor volume (p = 0.23) versus untreated control after 27 days. Longer-term safety assessments are ongoing in this model.
|
These data suggest that VAL-083 may be a viable treatment option for NSCLC patients failing TKI-therapy, especially where platinum-based therapy has already failed or is predicted to give sub-optimal outcomes. These results may have immediate implications in the treatment of NSCLC in China, where VAL-083 is approved for as a chemotherapy for the treatment of lung cancer. The data also support exploring future clinical development of VAL-083 as a lung cancer therapy in the rest of the world thereby providing DelMar with a potential opportunity to expand our clinical development focus beyond glioblastoma.
VAL-083 Target Markets
|
We are targeting cancer indications which we believe represent market opportunities in the hundreds of millions of dollars in North America and potentially in the billions of dollars worldwide. The pharmaceutical industry, in general, is a highly profitable, highly innovative industry. In 2006, the global pharmaceutical industry generated over $640 billion dollars in revenue. According to published reports, global pharmaceutical sales are highly stratified by region, with North America, the European Union and Japan accounting for 55% of global pharmaceutical sales in 2009; however, the most rapid growth in the sector is from developing countries, particularly China.
Glioblastoma Multiforme (GBM): Newly diagnosed patients suffering from GBM are initially treated through invasive brain surgery, although disease progression following surgical resection is nearly 100%. Temozolomide (Temodar ®) in combination with radiation is the front-line therapy for GBM following surgery. Temodar currently generates more than US$950 million annually in global revenues even though most patients fail to gain long-term therapeutic benefits. Approximately 60% of GBM patients treated with Temodar experience tumor progression within one year.
Bevacizumab (Avastin®) has been approved for the treatment of GBM in patients failing Temodar ®. In clinical studies, only about 20% of patients failing Temodar respond to Avastin therapy. In spite of these low efficacy results, treatment of GBM in North America alone is projected to add US$200 million annually to the revenues of Avastin with projected growth in GBM to US$650 million by 2016.
Approximately 48% of patients who are diagnosed with GBM will fail both front-line therapy and Avastin. Based on disease incidence, we believe the market for treating GBM patients the post-Avastin failure exceeds US$200 million annually in North America. Subject to successfully completing clinical trials and obtaining approval by the FDA and other applicable regulatory agencies globally, we also believe that VAL-083 could potentially generate sales in excess of $1 billion world-wide as a potential front-line therapy for GBM.
|
|
|
Leukemia: The potential of VAL-083 in the treatment of CML has been established in both human clinical trials conducted by the NCI and by the drug’s commercial approval in China. The Tyrosine Kinase Inhibitor Gleevec® is currently used as front-line therapy in the treatment of CML currently achieves global revenue in excess of $1 billion annually. We believe that VAL-083 has potential to capture a portion of the CML market through demonstration of activity in TKI-resistant CML patients. We also believe that VAL-083 may offer significant commercial opportunities through the treatment of other types of blood cancer such as AML or ALL.
Lung Cancer: The potential of VAL-083 in the treatment of NSLSC has been established in both human clinical trials conducted by the NCI and by the drug’s commercial approval in China. A 2012 report published by Decision Resources, Inc. (http://decisionresources.com/), forecasts that the NSCLC drug market will exceed US$4 billion in 2015.
VAL-083 Manufacturing
VAL-083 is currently manufactured in accordance with CFDA and Chinese Pharmacopoeia guidelines to ensure drug quality control, drug use safety, and drug efficacy. Approval by the FDA will require VAL-083 and other products developed by us to be manufactured in accordance with United States Pharmacopeia (“USP”) in accordance with Good Manufacturing Practices (“cGMP”) regulations. cGMP provides for systems that assure proper design, monitoring, and control of manufacturing processes and facilities. Adherence to the cGMP regulations assures the identity, strength, quality, and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations.
We have established an exclusive purchasing relationship with the Chinese manufacturer that has enabled us to obtain drug product for human clinical trials in the United States and certain commercial rights in China. The Chinese manufacturer has established a commercial-scale manufacturing process based on the North American process originally developed for the NCI.
Ensuring a viable long-term supply of the VAL-083 drug product suitable for registration and commercialization in North America and Europe will require investment in improved manufacturing and quality controls. We will seek to build upon our expertise and our intellectual property related to the existing manufacturing processes for VAL-083 in collaboration with the current manufacturer to allow compliance with cGMP. In addition, we have identified third party contract manufacturers with the capabilities to establish the processes, procedures and quality systems necessary to meet U.S., Canadian, E.U. and other international cGMP manufacturing requirements. Such requirements include strong quality management systems, obtaining appropriate quality raw materials, establishing robust operating procedures, detecting and investigating product quality deviations, and maintaining reliable testing laboratories.
Corporate Background
DelMar Pharmaceuticals, Inc. (the “Company”) is a Nevada corporation formed on June 24, 2009 under the name Berry Only Inc. (“Berry”). Prior to the Reverse Acquisition (discussed below), Berry did not have any significant assets or operations.
DelMar Pharmaceuticals, Inc. is the parent company of Del Mar Pharmaceuticals (BC) Ltd. (“DelMar (BC)”), a British Columbia, Canada corporation incorporated on April 6 , 2010, which is a clinical and commercial stage drug development company with a focus on the treatment of cancer. We are conducting clinical trials in the United States with our lead product, VAL-083, as a potential new treatment for glioblastoma multiforme (“GBM”), the most common and aggressive form of brain cancer. We have also acquired certain exclusive commercial rights to VAL-083 in China where it is approved as a chemotherapy for the treatment of chronic myelogenous leukemia (CML) and lung cancer. We plan to seek marketing partnerships in China in order to generate royalty revenue.
On January 25, 2013 (the “Closing Date”), the Company entered into and closed an exchange agreement (the “Exchange Agreement”), with DelMar (BC), 0959454 B.C. Ltd., a British Columbia corporation and a wholly-owned subsidiary of the Company (“Callco”), 0959456 B.C. Ltd., a British Columbia corporation and a wholly-owned subsidiary of the Company (“Exchangeco”), and securityholders of DelMar (BC). Pursuant to the Exchange Agreement, (i) the Company issued 4,340,417 shares of common stock (the “Parent Shares”) to the shareholders of DelMar (BC) who are United States residents (the “U.S. Holders”) in exchange for the transfer to Exchangeco of all 4,340,417 outstanding common shares of DelMar (BC) held by the U.S. Holders, (ii) the shareholders of DelMar (BC) who are Canadian residents (the “Canadian Holders”) received, in exchange for the transfer to Exchangeco of all 8,729,583 outstanding common shares of DelMar (BC) held by the Canadian Holders, 8,729,583 exchangeable shares (the “Exchangeable Shares”) of Exchangeco, and (iii) outstanding warrants to purchase 3,360,000 common shares of DelMar (BC) and outstanding options to purchase 1,020,000 common shares of DelMar (BC) were deemed to be amended such that, rather than entitling the holder to acquire common shares of DelMar (BC), such options and warrants (as amended, the “Exchange Agreement Warrants”) will entitle the holders to acquire shares of common stock of the Company. The Canadian Holders will be entitled to require Exchangeco to redeem (or, at the option of the Company or Callco, to have the Company or Callco purchase) the Exchangeable Shares, and upon such redemption or purchase to receive an equal number of shares of common stock of the Company.
Effective on the Closing Date, pursuant to the Exchange Agreement, DelMar (BC) became (indirectly through Exchangeco) a wholly-owned subsidiary of the Company. The acquisition of DelMar (BC) is treated as a reverse acquisition (the “Reverse Acquisition”), and the business of DelMar (BC) became the business of the Company. At the time of the Reverse Acquisition, Berry was not engaged in any active business.
We have incurred losses since our inception. Since our inception on April 6, 2010 through December 31, 2013, we have accumulated net losses of $15,684,506. We incurred net losses of $8,290,689 and $ 2,400,363 for the years ending December 31, 2013 and 2012, respectively.
Our executive offices are located at Suite 720-999 West Broadway, Vancouver, British Columbia, Canada V5Z 1K5. Our clinical operations are managed at Suite R, 3475 Edison Way, Menlo Park, California, 94025. Our website is located at www.delmarpharma.com, and our telephone number is 604-629-5989.
Directors and Executive Officers
The following persons are our executive officers, directors and/or control persons and hold the positions set forth opposite their name as of June 6, 2014:
| |
|
|
Name
|
Position(s)
|
|
|
Chairman of the Board, Chief Executive Officer, President, and Director
|
|
|
Chief Scientific Officer and Director
|
|
|
|
|
|
|
|
|
|
|
|
|
Each person’s address is c/o DelMar Pharmaceuticals, Inc., Suite 720-999 West Broadway, Vancouver, British Columbia, Canada V5Z 1K5.
SECTION 16. HISTORICAL AND PRO FORMA FINANCIAL INFORMATION REGARDING THE COMPANY
The Company has included its financial statements for the fiscal years ended December 31, 2013 and 2012 and for the quarterly period ended March 31, 2014 attached hereto as Exhibits A and B, respectively. The Company has included its pro forma information reflecting the effect of the Offer to Amend and Exercise attached hereto as Exhibit C. The Company’s book value per share as of March 31, 2014, based on 32,082,132 shares of common stock outstanding on such date, was ($0.095) per share.
In preparing this pro forma condensed financial data the Company assumed that all holders of Investor Warrants elected to participate in the Offer to Amend and Exercise for all 9,195,478 warrant shares eligible to participate in such Offer to Amend and Exercise and that the Offer to Amend and Exercise was completed as of the end of the quarterly period ended March 31, 2014. The pro forma condensed financial data is presented for informational and illustrative purposes only.
SECTION 17. INTERESTS OF DIRECTORS AND EXECUTIVE OFFICERS IN THE OFFER TO AMEND AND EXERCISE
None of the Company’s executive officers, directors or control persons hold Investor Warrants included in this offering.
SECTION 18. LEGAL MATTERS AND REGULATORY APPROVALS
We are not aware of any license or regulatory permit material to our business that might be adversely affected by the Offer to Amend and Exercise and the issuance of the shares of common stock upon the exercise of the Amended Warrants. Our obligations under the Offer to Amend and Exercise are subject to the conditions described in Section 6 “Conditions of the Offer to Amend and Exercise” above.
SECTION 19. MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES
The following is a summary of the material U.S. federal income tax consequences that we believe will be applicable to Investor Warrant holders who participate in the Offer to Amend and Exercise. However, we have not requested a ruling from the IRS or any opinion of counsel with regard to the treatment of warrant holders participating in the exchange and there can be no assurance, as discussed below, that the IRS will not take a position inconsistent with our expectations.
This discussion does not address all aspects of federal income taxation that may be relevant to you in light of your particular circumstances, or to those Investor Warrant holders who are subject to special rules, such as financial institutions and mutual funds; banks; insurance companies; investment companies; retirement plans; tax-exempt organizations; dealers or traders in securities; any person that holds their Investor Warrants as part of a straddle or hedge arrangement; partnerships or other pass-through entities; persons who are not citizens or residents of the United States or who are foreign corporations, foreign partnerships or foreign estates or trusts for U.S. federal income tax purposes or whose functional currency is not the U.S. dollar; or persons who are subject to the alternative minimum tax provisions of the Internal Revenue Code (the “ Code ”).
This discussion assumes that Investor Warrant holders hold the Investor Warrants as capital assets. In addition, the following discussion does not address the tax consequences of the participation in the Offer to Amend and Exercise under foreign, state or local tax laws. You are urged to consult your tax advisors as to the U.S. federal income tax consequences of participating in the Offer to Amend and Exercise and related reporting obligations, as well as the effects of state, local and non-U.S. tax laws and U.S. tax laws other than income tax laws.
Tax treatment of Investor Warrant holders participating in the Offer to Amend and Exercise.
Although not free from doubt, the Company intends to take the position that the amendment of your Investor Warrants followed by an exercise of the Amended Warrants is treated as an exchange of Investor Warrants for Amended Warrants which constitutes a recapitalization for U.S. federal income tax purposes, followed by the subsequent exercise of the Amended Warrants. Under this treatment, (i) an Investor Warrant holder who participates in the Offer to Amend would not recognize any gain or loss as a result of amending the Investor Warrants, (ii) such U.S. holder’s tax basis in the shares of our common stock received upon exercise of the Amended Warrants would be equal to the U.S. holder’s tax basis in the Investor Warrants plus the amount of any cash paid to exercise the Amended Warrants, and (iii) the holding period of the common stock would begin on the day after the exercise of the Amended Warrants.
Because of the lack of authority dealing with transactions similar to the Offer to Amend, the U.S. federal income tax consequences of the Offer to Amend are unclear, and alternative characterizations are possible that could require you to recognize gain or loss or may impact your holding period. The Internal Revenue Service has not made a determination, nor has the Company received any opinion of counsel, on the U.S. federal income tax consequences of the Offer to Amend or of a holder’s participation in the Offer to Amend. Therefore, we urge you to consult your tax advisor regarding the potential tax consequences of the Offer to Amend to you in your particular circumstances, including the consequences of possible alternative characterizations.
Distributions on Common Stock Received upon Exercise of New Warrants
After you exercise the Amended Warrant, any distributions you receive in respect of our common stock generally will be treated as a dividend, subject to tax as ordinary income, to the extent payable out of our current or accumulated earnings and profits (as determined for U.S. federal income tax purposes), then as a tax-free return of capital to the extent of your tax basis in the shares of our common stock, and thereafter as gain from the sale or exchange of the stock. Dividends received by a non-corporate holder currently qualify for taxation at a reduced 15% rate (subject to increase for tax years beginning after December 31, 2012) if the holder meets certain holding period and other applicable requirements. Dividends received by a corporate holder will be eligible for the dividends-received deduction if the holder meets certain holding period and other applicable requirements.
Sale or Other Taxable Disposition of Common Stock
You will generally recognize gain or loss upon the sale, exchange or other taxable disposition of shares of our common stock equal to the difference between (1) the amount of cash and the fair market value of any property received and (2) your adjusted tax basis in the shares of our common stock. Any gain or loss you recognize generally will be treated as a capital gain or loss. The capital gain or loss will be long-term if your holding period in the common stock is more than one year at the time of sale, exchange or other taxable disposition and will be short-term if your holding period is one year or less. Long-term capital gains of individuals and other non-corporate taxpayers are generally eligible for reduced rates of taxation. The deductibility of capital losses is subject to certain limitations.
Medicare Tax
For taxable years beginning after December 31, 2012, certain holders that are individuals, estates or trusts will be subject to a 3.8% Medicare tax on, among other things, dividends on and capital gains from the sale or other disposition of stock, subject to certain exceptions. You are urged to consult your tax advisors regarding the applicability of the Medicare tax to your income and gains arising from ownership and disposition of our common stock.
Information Reporting and Backup Withholding
Information reporting requirements generally will apply to certain holders with respect to dividends paid on, or, under certain circumstances, the proceeds of a sale, exchange or other disposition of, common stock. Under the Code and applicable Treasury Regulations, a holder of common stock may be subject to backup withholding (currently at a rate of 28%, subject to increase for taxable years beginning after December 31, 2012) with respect to dividends paid on common stock, or the proceeds of a sale, exchange or disposition of common stock, unless such holder (a) is a corporation or comes within certain other exempt categories and, when required, demonstrates this fact in the manner required, or (b) within a reasonable period of time, provides a correct taxpayer identification number, certifies that it is not subject to backup withholding and otherwise complies with applicable requirements of the backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules will generally be allowed as a credit against a holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided the required information is timely furnished to the IRS. You should consult their tax advisors regarding the application of information reporting and backup withholding rules in their particular situations, the availability of an exemption therefrom, and the procedure for obtaining such an exemption, if applicable.
Accounting Treatment
Under U.S. generally accepted accounting principles (“GAAP”), the anti-dilution provisions in the Investor Warrants causes the Investor Warrants to be treated as a derivative liability. As a result, we must record the Investor Warrants at their fair value on each balance sheet date and any change in value between reporting periods must be recorded as an incremental expense or income, as the case may be, for the period ending on such reporting date. The fair value of the derivative liability associated with the Investor Warrants increases as the price of our common stock increases, resulting in other expense in our consolidated statements of loss and comprehensive loss, and decreases as the price of our common stock decreases, resulting in other income. In other words, the existence of the anti-dilution provision causes our reported net loss to increase when the price of our common stock increases, and vice versa.
If the Investor Warrants are amended and exercised pursuant to the Offer to Amend and Exercise, this effect on our derivate liability will no longer occur for future periods for these warrants. In addition, upon the exercise of the Investor Warrants, the liability associated with the Investor Warrants would be reclassified from liabilities to stockholders’ equity, which would result in a decrease to the derivative liability account included in our balance sheet and an increase in stockholders’ equity.
SECTION 20. FEES AND EXPENSES
The Company has retained National Securities Corporation to act as its Warrant Agent for the Offer to Amend and Exercise pursuant to an Investment Banking Agreement, attached as Exhibits (d)(1) certain terms of which were extended on May 8, 2014 as set forth in Exhibit (d)(2) to its Schedule TO. National Securities Corporation, in accordance with the terms of the warrant agent engagement agreement shall use reasonable commercial efforts to contact holders of the Investor Warrants by mail, telephone, facsimile, or other electronic means and solicit their participation in the Offer to Amend and Exercise and to exercise their Amended Warrants. National Securities Corporation will receive a fee equal to 5% of the aggregate cash exercise price paid by holders of the Investor Warrants who participate in the Offer to Amend and Exercise. In addition, the Company has agreed to reimburse National Securities Corporation for its reasonable out-of-pocket expenses. If such expenses and fees exceed $1,000, National Securities Corporation] must thereafter provide invoices to the Company prior to seeking reimbursement and must obtain the Company’s prior approval. We have also issued National Securities Corporation a warrant to purchase 300,000 shares of the Company’s common stock at an exercise price of $1.76 per share. The warrant issued to National Securities terminates on September 12, 2018. The Company has agreed to indemnify National Securities Corporation against certain liabilities in connection with the Offer to Amend and Exercise, including certain liabilities under the federal securities laws.
The Company may also use the services of its officers and employees to solicit holders of the Investor Warrants to participate in the Offer to Amend and Exercise without additional compensation.
SECTION 21. TRANSFERS
The terms of the Investor Warrants provide that a holder may transfer the Investor Warrants to a third party if the transfer qualifies for an exemption from the registration requirements of the Securities Act to the reasonable satisfaction of the Company. Any holder of an Investor Warrant who desires to transfer an Investor Warrant should contact the Company prior to such transfer to ensure that the planned transfer satisfies the transfer restrictions set forth in the Investor Warrants.
SECTION 22. ADDITIONAL INFORMATION
The Company has filed with the SEC a Tender Offer Statement on Schedule TO of which this Offer to Amend and Exercise is a part. This Offer to Amend and Exercise does not contain all of the information contained in the Schedule TO and the exhibits to the Schedule TO. We recommend that holders of the Investor Warrants review the Schedule TO, including the exhibits, and the Company’s other materials that have been filed with the SEC before making a decision on whether to participate in the Offer to Amend and Exercise and to exercise the Amended Warrants.
The Board of Directors of the Company recognizes that the decision to participate in the Offer to Amend and Exercise and to exercise the Amended Warrants is an individual one that should be based on a variety of factors. The holders of the Investor Warrants should consult with their respective professional advisors if they have questions about their financial or tax situation. The information about this Offer to Amend and Exercise from the Company is limited to the Offering Materials.
The Company issued the Investor Warrants in private placement transactions in reliance on the exemption from registration provided by Rule 506 of Regulation D under the Securities Act of 1933, as amended (the “Securities Act”). In connection with such transactions, the holders of the Investor Warrants represented that they were “accredited investors.” The Company has included with this Offer to Amend and Exercise an exhibit titled “Supplemental Company Information” that contains additional information that holders of Investor Warrants, if any, who are no longer an “accredited investors” should consider before making an investment decision.
The Company is subject to the information requirements of the Securities Exchange Act of 1934, as amended, and in accordance therewith files and furnishes reports and other information with the SEC. All reports and other documents the Company has filed with the SEC, including the Schedule TO relating to the Offer to Amend and Exercise, or will file with the SEC in the future, can be accessed electronically on the SEC’s website at www.sec.gov .
SECTION 23. INFORMATION REQUESTS
Please direct questions or requests for assistance regarding this Offer to Amend and Exercise, Election to Participate and Exercise Warrant, and Notice of Withdrawal or other materials, in writing, to the Warrant Agent:
National Securities Corporation
Attn: Jonathan C. Rich
EVP - Director of Investment Banking
410 Park Avenue 14th Floor,
New York, NY, 10022
phone: 212-380-2819
fax: 212-380-2828
Please direct requests for additional copies of this Offer to Amend and Exercise, Election to Participate and Exercise Warrant, and Notice of Withdrawal or other materials, in writing, to the Company — DelMar Pharmaceuticals, Inc., ,Suite 720 - 999 West Broadway, Vancouver, British Columbia CANADA V5Z 1K5; Attn: Corporate Secretary.
| |
Sincerely,
|
|
| |
|
|
| |
/s/ Jeffrey A. Bacha
|
|
| |
Jeffrey A. Bacha
|
|
| |
Chief Executive Officer and President
|
|
| |
DelMar Pharmaceuticals, Inc.
|
|
| |
Suite 720 -- 999 West Broadway
|
|
| |
Vancouver, B.C. CANADA V5Z 1K5
|
|
| |
Phone: (604) 629-5989
|
|
DelMar Pharmaceuticals, Inc.
(a development stage company)
Consolidated Balance Sheets
As at December 31, 2013 and 2012
(in US dollars unless otherwise noted)
| |
|
Note
|
|
|
|
2013
$
|
|
|
|
2012
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
|
|
|
|
4,136,803 |
|
|
|
17,782 |
|
|
Taxes and other receivables
|
|
|
5 |
|
|
|
11,062 |
|
|
|
45,499 |
|
|
Prepaid expenses
|
|
|
|
|
|
|
170,883 |
|
|
|
28,778 |
|
|
Deferred costs
|
|
|
8 |
(f) |
|
|
- |
|
|
|
90,771 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
4,318,748 |
|
|
|
182,830 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities
|
|
|
6 |
|
|
|
140,457 |
|
|
|
677,615 |
|
|
Related party payables
|
|
|
9 |
|
|
|
109,030 |
|
|
|
447,777 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
249,487 |
|
|
|
1,125,392 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loan payable to Valent
|
|
|
4 |
|
|
|
272,372 |
|
|
|
264,352 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock option liability
|
|
|
8 |
|
|
|
212,561 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative liability
|
|
|
7 |
|
|
|
4,402,306 |
|
|
|
121,000 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
5,136,726 |
|
|
|
1,510,744 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Deficiency
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Authorized
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5,000,000 shares, $0.001 par value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 share outstanding at December 31, 2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(December 31, 2012 - nil)
|
|
|
8 |
|
|
|
- |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Authorized
|
|
|
|
|
|
|
|
|
|
|
|
|
|
200,000,000 shares, $0.001 par value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31,534,819 Issued at December 31, 2013 (December 31, 2012 - 13,050,000)
|
|
|
8 |
|
|
|
31,535 |
|
|
|
13,050 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
8 |
|
|
|
8,791,715 |
|
|
|
2,326,885 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants
|
|
|
8 |
|
|
|
6,202,100 |
|
|
|
153,106 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Deficit accumulated during the development stage
|
|
|
|
|
|
|
(15,864,506 |
) |
|
|
(3,842,133 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income
|
|
|
|
|
|
|
21,178 |
|
|
|
21,178 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
(817,978 |
) |
|
|
(1,327,914 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
4,318,748 |
|
|
|
182,830 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Nature of operations and going concern (note 1)
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (note 11)
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Subsequent events (note 13)
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Consolidated Statements of Operations and Comprehensive Loss
(in US dollars unless otherwise noted)
| |
|
Note
|
|
|
Year ended
December 31,
2013
$
|
|
|
Year ended
December 31,
2012
$
|
|
|
Year ended
December 31,
2011
$
|
|
|
Period from April 6, 2010 (inception) to
December 31,
2013
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
|
|
|
2,342,654 |
|
|
|
1,550,490 |
|
|
|
1,051,139 |
|
|
|
4,985,940 |
|
|
General and administrative
|
|
|
|
|
|
3,952,307 |
|
|
|
1,154,604 |
|
|
|
241,802 |
|
|
|
5,416,312 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
6,294,961 |
|
|
|
2,705,094 |
|
|
|
1,292,941 |
|
|
|
10,402,252 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other (income) loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of derivative liability
|
|
|
7 |
|
|
|
(1,324,051 |
) |
|
|
(318,502 |
) |
|
|
- |
|
|
|
(1,642,553 |
) |
|
Issuance of shares to Valent for future royalty reduction
|
|
|
|
|
|
|
598,000 |
|
|
|
- |
|
|
|
- |
|
|
|
598,000 |
|
|
Derivative issuance costs
|
|
|
|
|
|
|
2,713,220 |
|
|
|
24,742 |
|
|
|
- |
|
|
|
2,737,962 |
|
|
Foreign exchange loss (gain)
|
|
|
|
|
|
|
3,030 |
|
|
|
(18,492 |
) |
|
|
18,137 |
|
|
|
2,178 |
|
|
Interest expense
|
|
|
4 |
|
|
|
8,020 |
|
|
|
7,521 |
|
|
|
21,933 |
|
|
|
37,474 |
|
|
Interest income
|
|
|
|
|
|
|
(2,491 |
) |
|
|
- |
|
|
|
- |
|
|
|
(2,491 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
1,995,728 |
|
|
|
(304,731 |
) |
|
|
40,070 |
|
|
|
1,730,570 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the period
|
|
|
|
|
|
|
8,290,689 |
|
|
|
2,400,363 |
|
|
|
1,333,011 |
|
|
|
12,132,822 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share
|
|
|
|
|
|
|
(0.28 |
) |
|
|
(0.18 |
) |
|
|
(0.16 |
) |
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares
|
|
|
|
|
|
|
29,667,324 |
|
|
|
13,232,349 |
|
|
|
8,527,466 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
|
|
|
|
8,290,689 |
|
|
|
2,400,363 |
|
|
|
1,333,011 |
|
|
|
12,132,822 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive loss (income)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Translation to US dollar presentation currency
|
|
|
|
|
|
|
- |
|
|
|
21,121 |
|
|
|
(40,711 |
) |
|
|
(21,178 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss
|
|
|
|
|
|
|
8,290,689 |
|
|
|
2,421,484 |
|
|
|
1,292,300 |
|
|
|
12,111,644 |
|
The accompanying notes are an integral part of these consolidated financial statements.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Consolidated Statements of Changes in Stockholders' Deficiency
For the period from April 6, 2010 (inception) to December 31, 2013
(in US dollars unless otherwise noted)
| |
|
Number of
shares
|
|
|
Common
stock
$
|
|
|
Additional
paid-in
capital
$
|
|
|
Accumulated
other
comprehensive
income
$
|
|
|
Subscriptions
Receivable/ Warrants
$
|
|
|
Deficit
accumulated
during the
development
stage
$
|
|
|
Stockholders'
deficiency
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at April 6, 2010 (inception)
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of founders’ shares (note 8(a))
|
|
|
7,000,000 |
|
|
|
7,000 |
|
|
|
(333 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
6,667 |
|
|
Issuance of common shares (note 8(c))
|
|
|
1,000,000 |
|
|
|
1,000 |
|
|
|
94,403 |
|
|
|
- |
|
|
|
(28,506 |
) |
|
|
- |
|
|
|
66,897 |
|
|
Shares issued from Del Mar Employee Share Purchase Trust for services - net (note 8(b))
|
|
|
256,250 |
|
|
|
256 |
|
|
|
31,835 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
32,091 |
|
|
Comprehensive loss for the period
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,588 |
|
|
|
- |
|
|
|
- |
|
|
|
1,588 |
|
|
Loss for the period
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(108,759 |
) |
|
|
(108,759 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance - December 31, 2010
|
|
|
8,256,250 |
|
|
|
8,256 |
|
|
|
125,905 |
|
|
|
1,588 |
|
|
|
(28,506 |
) |
|
|
(108,759 |
) |
|
|
(1,516 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Collection of subscriptions receivable
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
28,506 |
|
|
|
- |
|
|
|
28,506 |
|
|
Issuance of units net of cash issue costs (note 7)
|
|
|
400,000 |
|
|
|
400 |
|
|
|
119,496 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
119,896 |
|
|
Issuance of units for services (notes 7 and 9)
|
|
|
200,000 |
|
|
|
200 |
|
|
|
60,101 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
60,301 |
|
|
Issuance of units for settlement of accounts payable (notes 7 and 9)
|
|
|
50,000 |
|
|
|
50 |
|
|
|
15,025 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
15,075 |
|
|
Issuance of warrants related to share issuance costs of units (notes 7 and 8)
|
|
|
- |
|
|
|
- |
|
|
|
8,333 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
8,333 |
|
|
Issuance of warrants for patents (notes 4 and 8)
|
|
|
- |
|
|
|
- |
|
|
|
89,432 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
89,432 |
|
|
Shares issued from Del Mar Employee Share Purchase Trust for services - net (note 8(b))
|
|
|
153,125 |
|
|
|
153 |
|
|
|
94,987 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
95,140 |
|
|
Comprehensive loss for the year
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
40,711 |
|
|
|
- |
|
|
|
- |
|
|
|
40,711 |
|
|
Loss for the year
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(1,333,011 |
) |
|
|
(1,333,011 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance - December 31, 2011
|
|
|
9,059,375 |
|
|
|
9,059 |
|
|
|
513,279 |
|
|
|
42,299 |
|
|
|
- |
|
|
|
(1,441,770 |
) |
|
|
(877,133 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Consolidated Statements of Changes in Stockholders' Deficiency …continued
For the period from April 6, 2010 (inception) to December 31, 2013
(in US dollars unless otherwise noted)
| |
|
Number of
shares
|
|
|
Common
stock
$
|
|
|
Additional
paid-in
capital
$
|
|
|
Accumulated
other
comprehensive
income
$
|
|
|
Subscriptions
Receivable/ Warrants
$
|
|
|
Deficit
accumulated
during the
development
stage
$
|
|
|
Stockholders'
deficiency
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance - December 31, 2011
|
|
|
9,059,375 |
|
|
|
9,059 |
|
|
|
513,279 |
|
|
|
42,299 |
|
|
|
- |
|
|
|
(1,441,770 |
) |
|
|
(877,133 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of units net of cash issue costs (note 7)
|
|
|
4,400,000 |
|
|
|
4,400 |
|
|
|
1,358,172 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,362,572 |
|
|
Issuance of units for services (notes 7 and 9)
|
|
|
360,000 |
|
|
|
360 |
|
|
|
116,915 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
117,275 |
|
|
Units cancelled (note 7)
|
|
|
(3,000,000 |
) |
|
|
(3,000 |
) |
|
|
(938,813 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(941,813 |
) |
|
Reclassification from additional paid-in capital to warrants upon the issuance of warrants
(note 8)
|
|
|
- |
|
|
|
- |
|
|
|
(103,727 |
) |
|
|
- |
|
|
|
103,727 |
|
|
|
- |
|
|
|
- |
|
|
Issuance of warrants for services (notes 8)
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
49,379 |
|
|
|
- |
|
|
|
49,379 |
|
|
Issuance of shares for settlement of accounts payable (notes 8 and 9)
|
|
|
500,000 |
|
|
|
500 |
|
|
|
252,550 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
253,050 |
|
|
Shares issued from Del Mar Employee Share Purchase Trust for services - net (note 8(b))
|
|
|
1,590,625 |
|
|
|
1,591 |
|
|
|
780,255 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
781,846 |
|
|
Shares issued for services (note 8(h))
|
|
|
140,000 |
|
|
|
140 |
|
|
|
75,660 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
75,800 |
|
|
Stock-based compensation (note 8)
|
|
|
- |
|
|
|
- |
|
|
|
272,594 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
272,594 |
|
|
Comprehensive income for the year
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(21,121 |
) |
|
|
- |
|
|
|
- |
|
|
|
(21,121 |
) |
|
Loss for the year
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(2,400,363 |
) |
|
|
(2,400,363 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance - December 31, 2012
|
|
|
13,050,000 |
|
|
|
13,050 |
|
|
|
2,326,885 |
|
|
|
21,178 |
|
|
|
153,106 |
|
|
|
(3,842,133 |
) |
|
|
(1,327,914 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of the Reverse Acquisition (note 3)
|
|
|
3,250,007 |
|
|
|
3,250 |
|
|
|
1,686,754 |
|
|
|
- |
|
|
|
- |
|
|
|
(3,731,684 |
) |
|
|
(2,041,680 |
) |
|
Issuance of units at $0.80 per unit from January 25 to March 6, 2013, net of cash issue costs (note 8(f))
|
|
|
13,125,002 |
|
|
|
13,125 |
|
|
|
5,854,252 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
5,867,377 |
|
|
Issuance of placement agent warrants as issue costs for the $0.80 unit issuance
(note 8(f))
|
|
|
- |
|
|
|
- |
|
|
|
(4,087,586 |
) |
|
|
- |
|
|
|
6,288,594 |
|
|
|
- |
|
|
|
2,201,008 |
|
|
Issuance of common shares to Valent for future royalty reduction (note 8 (g))
|
|
|
1,150,000 |
|
|
|
1,150 |
|
|
|
596,850 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
598,000 |
|
|
Exercise of placement agent warrants (note 8)
|
|
|
123,810 |
|
|
|
124 |
|
|
|
239,476 |
|
|
|
- |
|
|
|
(239,600 |
) |
|
|
- |
|
|
|
- |
|
|
Exercise of CDN $0.50 unit warrants (notes 7 and 8)
|
|
|
221,000 |
|
|
|
221 |
|
|
|
241,494 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
241,715 |
|
|
Shares issued for services
(note 8(h))
|
|
|
615,000 |
|
|
|
615 |
|
|
|
1,042,942 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,043,557 |
|
|
Stock-based compensation
(note 8)
|
|
|
- |
|
|
|
- |
|
|
|
890,648 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
890,648 |
|
|
Loss for the period
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(8,290,689 |
) |
|
|
(8,290,689 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance – December 31, 2013
|
|
|
31,534,819 |
|
|
|
31,535 |
|
|
|
8,791,715 |
|
|
|
21,178 |
|
|
|
6,202,100 |
|
|
|
(15,864,506 |
) |
|
|
(817,978 |
) |
DelMar Pharmaceuticals, Inc.
(a development stage company)
Consolidated Statements of Cash Flows
(in US dollars unless otherwise noted)
| |
|
Year ended
December 31,
2013
$
|
|
|
Year ended
December 31,
2012
$
|
|
|
Year ended
December 31,
2011
$
|
|
|
Period from April 6, 2010 (inception) to
December 31,
2013
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss for the year
|
|
|
(8,290,689 |
) |
|
|
(2,400,363 |
) |
|
|
(1,333,011 |
) |
|
|
(12,132,822 |
) |
|
Items not affecting cash
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accrued interest
|
|
|
8,020 |
|
|
|
7,521 |
|
|
|
6,831 |
|
|
|
22,372 |
|
|
Change in fair value of derivative liability
|
|
|
(1,324,051 |
) |
|
|
(318,502 |
) |
|
|
- |
|
|
|
(1,642,553 |
) |
|
Shares issued to Valent for royalty reduction
|
|
|
598,000 |
|
|
|
- |
|
|
|
- |
|
|
|
598,000 |
|
|
Non-cash derivative issue costs
|
|
|
2,201,008 |
|
|
|
- |
|
|
|
- |
|
|
|
2,201,008 |
|
|
Units issued for services
|
|
|
- |
|
|
|
180,144 |
|
|
|
95,140 |
|
|
|
275,284 |
|
|
Warrants issued for patents
|
|
|
- |
|
|
|
- |
|
|
|
89,432 |
|
|
|
89,432 |
|
|
Warrants issued for services
|
|
|
124,020 |
|
|
|
49,379 |
|
|
|
- |
|
|
|
173,399 |
|
|
Share-based compensation
|
|
|
2,146,766 |
|
|
|
1,130,240 |
|
|
|
95,140 |
|
|
|
3,404,237 |
|
|
Prototype drug product
|
|
|
- |
|
|
|
- |
|
|
|
250,000 |
|
|
|
250,000 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
(4,536,926 |
) |
|
|
(1,351,581 |
) |
|
|
(796,468 |
) |
|
|
(6,761,643 |
) |
|
Changes in non-cash working capital
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Taxes and other receivables
|
|
|
34,437 |
|
|
|
(6,697 |
) |
|
|
(24,017 |
) |
|
|
(11,062 |
) |
|
Prepaid expenses
|
|
|
(142,105 |
) |
|
|
(14,581 |
) |
|
|
(4,098 |
) |
|
|
(170,883 |
) |
|
Accounts payable and accrued liabilities
|
|
|
(537,158 |
) |
|
|
865,007 |
|
|
|
99,297 |
|
|
|
367,375 |
|
|
Related party payables
|
|
|
(338,747 |
) |
|
|
(70,183 |
) |
|
|
496,597 |
|
|
|
109,030 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
(5,520,499 |
) |
|
|
(578,035 |
) |
|
|
(228,689 |
) |
|
|
(6,467,183 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net proceeds from the issuance of units
|
|
|
9,639,520 |
|
|
|
671,570 |
|
|
|
190,826 |
|
|
|
10,501,916 |
|
|
Deferred costs
|
|
|
- |
|
|
|
(90,771 |
) |
|
|
- |
|
|
|
- |
|
|
Net proceeds from the issuance of common shares
|
|
|
- |
|
|
|
- |
|
|
|
28,506 |
|
|
|
102,070 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
9,639,520 |
|
|
|
580,799 |
|
|
|
219,332 |
|
|
|
10,603,986 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase (decrease) in cash and cash equivalents
|
|
|
4,119,021 |
|
|
|
2,764 |
|
|
|
(9,357 |
) |
|
|
4,136,803 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents - beginning of period
|
|
|
17,782 |
|
|
|
15,018 |
|
|
|
24,375 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents - end of period
|
|
|
4,136,803 |
|
|
|
17,782 |
|
|
|
15,018 |
|
|
|
4,136,803 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplementary information
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of shares for the settlement of accounts payable (notes 4 and 9)
|
|
|
- |
|
|
|
253,050 |
|
|
|
- |
|
|
|
253,050 |
|
|
Issuance of units for the settlement of accounts payable (notes 7 and 9)
|
|
|
- |
|
|
|
- |
|
|
|
23,785 |
|
|
|
23,785 |
|
|
Non-cash share issuance costs (note 8)
|
|
|
6,288,594 |
|
|
|
- |
|
|
|
14,295 |
|
|
|
6,302,889 |
|
|
Cashless exercise of Placement Agent Warrants (note 8)
|
|
|
239,600 |
|
|
|
- |
|
|
|
- |
|
|
|
239,600 |
|
|
Non-cash acquisition of prototype drug product (note 4)
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
250,000 |
|
|
Settlement of accounts payable with a loan payable (note 4)
|
|
|
- |
|
|
|
- |
|
|
|
250,000 |
|
|
|
250,000 |
|
|
Exercise of CDN $0.50 warrants for no additional consideration (note 8)
|
|
|
241,715 |
|
|
|
- |
|
|
|
- |
|
|
|
241,715 |
|
|
Deferred costs
|
|
|
90,771 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
The accompanying notes are an integral part of these consolidated financial statements.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
|
1
|
Nature of operations and going concern
|
Nature of operations
DelMar Pharmaceuticals, Inc. (the “Company”) is a Nevada corporation formed on June 24, 2009 under the name Berry Only Inc. Prior to the Reverse Acquisition (note 3), Berry did not have any significant assets or operations. DelMar Pharmaceuticals, Inc. is the parent company of Del Mar Pharmaceuticals (BC) Ltd. (“DelMar (BC)”), a British Columbia, Canada corporation incorporated on April 6, 2010, which is a development stage company with a focus on the development of drugs for the treatment of cancer. It is also the parent company to 0959454 B.C. Ltd., a British Columbia corporation (“Callco”), and 0959456 B.C. Ltd., a British Columbia corporation (“Exchangeco”). Callco and Exchangeco were formed to facilitate the Reverse Acquisition (note 3).
Pursuant to the Reverse Acquisition, the Company acquired (either directly or indirectly (through Exchangeco)) all of the issued and outstanding shares of DelMar (BC) on January 25, 2013 (note 3). As a result of the shareholders of DelMar (BC) having a controlling interest in the Company subsequent to the Reverse Acquisition, for accounting purposes the transaction is a capital transaction with DelMar (BC) being the accounting acquirer even though the legal acquirer is Berry. Therefore, the historic financial statements of DelMar (BC) are presented as the comparative balances for the periods prior to the Reverse Acquisition.
References to the Company refer to the Company and its wholly-owned subsidiaries, DelMar (BC), Callco and Exchangeco. References to Berry relate to the Company prior to the Reverse Acquisition.
The Company is a development stage company focused on the discovery and development of new medicines with the potential to treat cancer patients who have failed modern targeted or biologic therapy. The Company has initiated a clinical trial with its lead drug candidate VAL-083 for the treatment of refractory glioblastoma multiforme (“GBM”). The Phase I/II study is an open-label, single arm dose-escalation study designed to evaluate the safety, tolerability, pharmacokinetics and anti-tumor activity of VAL-083 in patients with histologically confirmed initial diagnosis of primary WHO Grade IV malignant glioma, now recurrent. Patients with prior low-grade glioma or anaplastic glioma are eligible, if histologic assessment demonstrates transformation to GBM.
The address of the Company’s administrative offices is Suite 720 - 999 West Broadway, Vancouver, British Columbia, V5Z 1K5 with clinical operations located at 3485 Edison Way, Suite R, Menlo Park, California, 94025.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
Going Concern
For the year ended December 31, 2013, the Company reported a loss of $8,290,689 and an accumulated deficit of $15,864,506 at that date. As at December 31, 2013, the Company has cash and cash equivalents on hand of $4,136,803. The Company does not have the prospect of achieving revenues in the near future and the Company will require additional funding to maintain its research and development projects and for general operations. These circumstances lend substantial doubt as to the ability of the Company to meet its obligations as they come due. The expenses to be incurred in developing and pursuing our Company’s business plan have a large degree of uncertainty. In addition, the Company has not begun to commercialize or generate revenues from any product candidate.
Consequently, management is pursuing various financing alternatives to fund the Company’s operations so it can continue as a going concern in the medium to longer term. Accordingly, the Company is considered to be in the development stage as defined in Accounting Standards Codification (ASC) 915-10. We believe, based on our current estimates and plans that we have enough cash to fund our operations for the next 12 to 15 months. Management plans to secure the necessary financing through the issue of new equity and/or the entering into of strategic partnership arrangements. Nevertheless, there is no assurance that these initiatives will be successful.
These financial statements have been prepared on a going concern basis which assumes that the Company will continue its operations for the foreseeable future and contemplates the realization of assets and the settlement of liabilities in the normal course of business.
The conditions and risks noted above cast substantial doubt on the validity of that assumption. These financial statements do not give effect to any adjustments to the amounts and classification of assets and liabilities that may be necessary and could potentially be material, should the Company be unable to continue as a going concern.
|
2
|
Significant accounting policies
|
Basis of presentation
The financial statements of the Company have been prepared in accordance with United States Generally Accepted Accounting Principles (“US GAAP”) and are presented in United States dollars. The Company’s functional currency is the United States dollar.
The principal accounting policies applied in the preparation of these financial statements are set out below and have been consistently applied to all periods presented.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
Consolidation
The consolidated financial statements include the accounts of Del Mar Pharmaceuticals (BC) Ltd., Callco, and Exchangeco as of and for the years ended December 31, 2013, 2012 and 2011. Inter-company transactions have been eliminated in consolidation.
Use of estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions about future events that affect the reported amounts of assets, liabilities, expenses, contingent assets and contingent liabilities as at the end or during the reporting period. Actual results could significantly differ from those estimates. Significant areas requiring management to make estimates include the derivative liability and the valuation of equity instruments issued for services. Further details of the nature of these assumptions and conditions may be found in the relevant notes to the financial statements.
|
a)
|
Fair value of derivative liability
|
The derivative is not traded in an active market and the fair value is determined using valuation techniques. The Company uses judgment to select a variety of methods to make assumptions that are based on specific management plans and market conditions at the end of each reporting period. The Company uses a fair value estimate to determine the fair value of the derivative liability. The carrying value of the derivative liability would be higher or lower as management estimates around specific probabilities change. The estimates may be significantly different from those recorded in the financial statements because of the use of judgment and the inherent uncertainty in estimating the fair value of these instruments that are not quoted in an active market. All changes in the fair value are recorded in the consolidated statement of loss each reporting period. This is considered to be a Level 3 financial instrument.
Cash and cash equivalents
Cash and cash equivalents consist of cash on deposit and highly liquid short-term interest-bearing securities with maturities at the date of purchase of three months or less. Cash and cash equivalents are held at recognized Canadian and United States financial institutions. Interest earned is recognized in the consolidated statements of loss. In 2013 the Company raised financing of net proceeds of $8,575,000. The use of proceeds under this arrangement stated that the proceeds from the financing cannot be used to repay debt.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
Foreign currency translation
The functional currency of the Company at December 31, 2013 is the United States dollar. Transactions that are denominated in a foreign currency are re-measured into the functional currency at the current exchange rate on the date of the transaction. Any foreign-currency denominated monetary assets and liabilities are subsequently re-measured at current exchange rates, with gains or losses recognized as foreign exchange losses or gains in the consolidated statement of operations. Nonmonetary assets and liabilities are translated at historical exchange rates. Expenses are translated at average exchange rates during the period. Exchange gains and losses are included in consolidated statement of operations for the period.
Adjustments arising from the translation of the Company’s financial statements to United States dollars for the periods ended December 31, 2012, 2011 and 2010 due to differences between average rates and balance sheet rates are recorded in other comprehensive income as for those periods the Company’s functional currency was the Canadian dollar. For those periods the financial statements have been presented in a currency other than the functional currency of the Company as management has determined that the U.S. dollar is the common currency in which the Company’s peers, being international drug and pharmaceutical companies, present their financial statements. For presentation purposes the assets and liabilities of the Company for 2012, 2011, and 2010 have been translated to U.S. dollars at exchange rates at the reporting date. The historical equity transactions have been translated using historical rates in effect on the date that each transaction occurred. The income and expenses are translated to U.S. dollars at the average exchange rate for the period in which the transaction arose. Exchange differences arising are recognized in a separate component of equity titled accumulated other comprehensive income.
Current and future income taxes
The Company follows the liability method of accounting for income taxes. Under this method, current income taxes are recognized for the estimated income taxes payable for the current period. Income taxes are accounted for using the asset and liability method of accounting. Future income taxes are recognized for the future income tax consequences attributable to differences between the carrying values of assets and liabilities and their respective income tax bases and for loss carry-forwards. Future income tax assets and liabilities are measured using enacted income tax rates expected to apply to taxable income in the periods in which temporary differences are expected to be recovered or settled. The effect on future income tax assets and liabilities of a change in tax laws or rates is included in earnings in the period that includes the enactment date. When realization of future income tax assets does not meet the more-likely-than-not criterion for recognition, a valuation allowance is provided.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
Financial instruments
The Company has financial instruments that are measured at fair value. To determine the fair value, we use the fair value hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use to value an asset or liability and are developed based on market data obtained from independent sources. Unobservable inputs are inputs based on assumptions about the factors market participants would use to value an asset or liability. The three levels of inputs that may be used to measure fair value are as follows:
|
·
|
Level one - inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities;
|
|
·
|
Level two - inputs are inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly such as interest rates, foreign exchange rates, and yield curves that are observable at commonly quoted intervals; and
|
|
·
|
Level three - unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use.
|
Assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. Changes in the observability of valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy.
The Company’s financial instruments consist of cash and cash equivalents, taxes and other receivables, accounts payable, related party payables and derivative liability. The carrying values of cash and cash equivalents, taxes and other receivables, accounts payable and related party payables approximate their fair values due to the immediate or short-term maturity of these financial instruments.
As quoted prices for the derivative liability are not readily available, the Company has used a simulated probability valuation model, as described in note 7 to estimate fair value. The derivative liability utilizes Level 3 inputs as defined above.
The Company has the following liabilities under the fair value hierarchy:
| |
|
|
|
|
|
|
|
2013
|
|
| |
|
|
|
|
|
|
|
|
|
|
Liability
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
| |
|
|
|
|
|
|
|
|
|
|
Derivative liability
|
|
|
- |
|
|
|
- |
|
|
|
4,402,306 |
|
| |
|
|
|
|
|
|
|
2012
|
|
| |
|
|
|
|
|
|
|
|
|
|
Liability
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
| |
|
|
|
|
|
|
|
|
|
|
Derivative liability
|
|
|
- |
|
|
|
- |
|
|
|
121,000 |
|
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
Prototype drug product
The prototype drug product (the “drug”) is stated at the lower of cost and net realizable value. The cost of the drug is comprised of direct costs related to the acquisition of the drug. During the years ended 2012 and 2011, the Company recorded $nil in relation to these amounts as inventories (2010 - $250,000 was recorded as prototype drug product) and fully utilized in clinical and pre-clinical testing trials during the year ended December 31, 2011.
Intangible assets
Under its assignment agreement with Valent Technologies LLC (“Valent”) (note 4) the Company has incurred certain costs relating to patents assigned to the Company. As the patents had not yet been assigned to the Company at December 31, 2011, the Company has expensed these costs for the year ended December 31, 2011.
Expenditures associated with the filing, or maintenance of patents, licensing or technology agreements are expensed as incurred. Costs previously recognized as an expense are not recognized as an asset in subsequent periods.
Once the technology has achieved commercialization, patent costs will be deferred and amortized over the remaining life of the related patent.
Research and development costs (including clinical trial expenses)
Research and development expenses include payroll, employee benefits, stock-based compensation expense, and other headcount-related expenses associated with product research and development. Research and development expenses also include third-party development and clinical trial expenses noted below. Such costs related to product research and development are included in research and development expense until the point that technological feasibility is reached, which for our products, is generally shortly before the products are approved by the relevant food and drug administration. Once technological feasibility is reached, such costs are capitalized and amortized to cost of revenue over the estimated lives of the products.
Clinical trial expenses are a component of research and development costs and include fees paid to contract research organizations, investigators and other service providers who conduct specific research for product development activities on behalf of the Company. The amount of clinical trial expenses recognized in a period related to service agreements is based on estimates of the work performed on an accrual basis. These estimates are based on patient enrollment, services provided and goods delivered, contractual terms and experience with similar contracts. The Company monitors these factors to the extent possible and adjusts our estimates accordingly. Prepaid expenses or accrued liabilities are adjusted if payments to service providers differ from estimates of the amount of service completed in a given period.
Research and development costs are expensed in the period incurred. At December 31, 2013 and 2012 all research and development costs were expensed.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
Shares for services
The Company has issued equity instruments for services provided by employees and nonemployees. The equity instruments are valued at the fair value of the instrument granted (see notes 7 and 8 for assumptions).
The Company has transferred shares from the DelMar Employee Share Purchase Trust (the “Trust”) (note 8) to consultants and management in exchange for services rendered to the Company. The Company recognizes the fair value of the shares transferred as an expense with a corresponding increase in common stock. The shares reserved for issuance to consultants and management that are held by the Trust are included in the financial statements at year end. There are no other assets in the Trust. The number of shares outstanding for issue from the Trust at December 31, 2013 is nil (2012 - nil; 2011 - 1,590,625) (note 8).
The shares transferred from the Trust have been valued using the fair value of the shares transferred. The Company used recent share transactions in order to determine the fair value of the shares transferred from the Trust.
Stock options
The Company accounts for these awards under ASC 718, “Compensation - Stock Compensation” (“ASC 718”). ASC 718 requires measurement of compensation cost for all stock-based awards at fair value on the date of grant and recognition of compensation over the requisite service period for awards expected to vest. Compensation expense for unvested options to non-employees is revalued at each period end and is being amortized over the vesting period of the options. The determination of grant-date fair value for stock option awards is estimated using the Black-Scholes model, which includes variables such as the expected volatility of the Company’s share price, the anticipated exercise behavior of its grantee, interest rates, and dividend yields. These variables are projected based on the Company’s historical data, experience, and other factors. Changes in any of these variables could result in material adjustments to the expense recognized for share-based payments. Such value is recognized as expense over the requisite service period, net of estimated forfeitures, using the straight-line attribution method. The estimation of stock awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from current estimates, such amounts are recorded as a cumulative adjustment in the period estimates are revised. The Company considers many factors when estimating expected forfeitures, including type of awards granted, employee class, and historical experience. Actual results and future estimates may differ substantially from current estimates.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
Comprehensive income
In accordance with ASC 220, “Comprehensive Income” (“ASC 220”) all components of comprehensive income, including net loss, are reported in the financial statements in the period in which they are recognized. Comprehensive income is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Net loss and other comprehensive (income) loss, , including foreign currency translation adjustments, are reported, net of any related tax effect, to arrive at comprehensive income. No taxes were recorded on items of other comprehensive income.
Derivative liability
The Company accounts for certain warrants under the authoritative guidance on accounting for derivative financial instruments indexed to, and potentially settled in, a company’s own stock, on the understanding that in compliance with applicable securities laws, the warrants require the issuance of securities upon exercise and do not sufficiently preclude an implied right to net cash settlement. The Company classifies these warrants on its balance sheet as a derivative liability which is fair valued at each reporting period subsequent to the initial issuance. The Company has used a simulated probability valuation model to value the warrants. Determining the appropriate fair-value model and calculating the fair value of warrants requires considerable judgment. Any change in the estimates (specifically probabilities) used may cause the value to be higher or lower than that reported. The estimated volatility of the Company’s common stock at the date of issuance, and at each subsequent reporting period, is based on the historical volatility of similar life sciences companies. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the warrants at the valuation date. The expected life of the warrants is assumed to be equivalent to their remaining contractual term.
Loss per share
Income or loss per share is calculated based on the weighted average number of common shares outstanding. Diluted loss per share does not differ from basic loss per share since the effect of the Company’s warrants and stock options are anti-dilutive. Diluted income per share is calculated using the treasury stock method which uses the weighted average number of common shares outstanding during the period and also includes the dilutive effect of potentially issuable common shares from outstanding stock options and warrants. At December 31, 2013, potential common shares of 28,104,009 (2012 - 4,380,000; 2011 - 650,000) related to outstanding warrants and stock options were excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive.
Segment information
The Company identifies its operating segments based on business activities, management responsibility and geographical location. The Company operates within a single operating segment being the research and development of cancer indications, and operates in one geographic area, being Canada. All of the Company’s assets are located in Canada.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
Recent accounting pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies that are adopted by the Company as of the specified effective date. Unless otherwise discussed, we believe that the impact of recently issued standards that are not yet effective will not have a material impact on our financial position or results of operations upon adoption.
ASU 2013-07 Topic 205 Liquidation basis of accounting
Provides guidance on (i) when an entity should apply the liquidation basis of accounting, and (ii) recognition and measurement of assets and liabilities, and requirements for preparation of financial statements, using the liquidation basis of accounting.
This standard is effective for entities that determine liquidation is imminent during years, and interim periods within those years, beginning after December 15, 2013.
ASU 3013-05 Topic 830 Accounting for cumulative translation adjustments
The standards amends cumulative translation adjustment derecognition guidance in particular when (i) an entity ceases to have a controlling financial interest in certain subsidiaries or groups of assets within a foreign entity, or (ii) there is a loss of a controlling financial interest in a foreign entity or a step acquisition involving an equity method investment that is a foreign entity. This is effective for public entities for years, and interim periods within those years, beginning after December 15, 2013.
ASU 2013-11 Topic 740 Accounting for cumulative translation adjustments
The standard amends guidance on financial statement presentation of an unrecognized tax benefit when a net operating loss carryforward, a similar tax loss, or a tax credit carryforward exists. This is effective for public entities for years, and interim periods within those years, beginning after December 15, 2013.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
On January 25, 2013 (the “Closing Date”), the Company entered into and closed an exchange agreement (the “Exchange Agreement”), with DelMar (BC), Callco, Exchangeco, and the securityholders of DelMar (BC). Pursuant to the Exchange Agreement, (i) the Company issued 4,340,417 shares of common stock (the “Parent Shares”) to the shareholders of DelMar (BC) who are United States residents (the “U.S. Holders”) in exchange for the transfer to Exchangeco of all 4,340,417 outstanding common shares of DelMar (BC) held by the U.S. Holders, (ii) the shareholders of DelMar (BC) who are Canadian residents (the “Canadian Holders”) received, in exchange for the transfer to Exchangeco of all 8,729,583 outstanding common shares of DelMar (BC) held by the Canadian Holders, 8,729,583 exchangeable shares (the “Exchangeable Shares”) of Exchangeco, and (iii) outstanding warrants to purchase 3,360,000 common shares of DelMar (BC) and outstanding options to purchase 1,020,000 common shares of DelMar (BC) were deemed to be amended such that, rather than entitling the holder to acquire common shares of DelMar (BC), such options and warrants will entitle the holders to acquire shares of common stock of the Company. The Canadian Holders will be entitled to require Exchangeco to redeem (or, at the option of the Company or Callco, to have the Company or Callco purchase) the Exchangeable Shares, and upon such redemption or purchase to receive an equal number of shares of common stock of the Company. The aggregate of 13,070,000 shares of common stock of the Company issued to the former shareholders of DelMar (BC) (on an as-exchanged basis with respect to the Exchangeable Shares) represents 80.1% of the outstanding shares of common stock of the Company following the closing of the Exchange Agreement (the “Reverse Acquisition”).
Upon completion of the Reverse Acquisition DelMar (BC) became a wholly-owned subsidiary of the Company. As a result of the shareholders of DelMar (BC) having a controlling interest in the Company subsequent to the Reverse Acquisition, for accounting purposes the transaction is a capital transaction with DelMar (BC) being the accounting acquirer even though the legal acquirer is Berry. No goodwill is recorded with respect to the transaction as it does not constitute a business combination. For accounting purposes, the transaction is reflected as a recapitalization of DelMar (BC) and consideration for the Reverse Acquisition was deemed to be the fair value of the shares that were issued by DelMar (BC) to acquire the net liabilities of Berry on January 25, 2013. The net identifiable liabilities of Berry on the Closing Date of the Reverse Acquisition were as follows:
| |
|
$ |
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Net liabilities (derivative liability)
|
|
|
2,041,680 |
|
|
|
|
|
The Company determined the fair value of the shares issued on the Reverse Acquisition to be $1,690,004. As a result of the Reverse Acquisition being treated as a recapitalization of DelMar (BC) the Company recognized the loss of $3,731,684 incurred upon the closing of the Reverse Acquisition as an adjustment to opening deficit in the consolidated statement of changes in stockholders’ deficiency at December 31, 2013.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
|
4
|
Valent Technologies LLC agreement
|
On September 12, 2010 the Company entered into a Patent Assignment Agreement (the “Assignment Agreement”) with Valent Technologies LLC (“Valent”) to acquire patents and the prototype drug product related to VAL-083. In accordance with the Assignment Agreement the consideration was $250,000 to acquire the prototype drug product. In addition, under certain circumstances Valent agreed to assign, convey and transfer to the Company all its right, title and interest in and to the patents in exchange for share purchase warrants. The Company will then be responsible for the further development and commercialization of VAL-083. Valent retains an option to reacquire certain intellectual property until a Financing Transaction is completed by the Company. Under the Assignment Agreement, a ‘Financing Transaction’ is defined as a cumulative equity or debt financing(s), or a merger, acquisition, amalgamation, reverse takeover or other combination, or any combination of the foregoing, cumulatively totaling at least $2,000,000. In accordance with the terms of the Assignment Agreement, Valent is entitled to receive a future royalty on revenues derived from the development and commercialization of VAL-083. In the event that the Company terminates the agreement, the Company may be entitled to receive royalties from Valent’s subsequent development of VAL-083 depending on the development milestones the Company has achieved prior to the termination of the Assignment Agreement.
On January 25, 2013, in connection with the Company’s reverse acquisition, Valent was issued 1,150,000 shares of common stock of DelMar Pharmaceuticals, Inc., in exchange for Valent reducing certain future royalties under the Assignment Agreement.
Pursuant to a loan agreement dated February 3, 2011, the Company received a loan from Valent for the $250,000 for the purchase of the prototype drug product. The loan is unsecured and bears interest at 3.00% per year. As a result the balance of the loan payable, including accrued interest, at December 31, 2013 is $272,372 (2012 - $264,352), including accrued interest of $22,372 (2012 - $14,352). As a result of the Company’s expectation as to the timing of repayment as a result of the restriction described in note 2 the Company has presented the full loan and accrued interest balance as a non-current liability at December 31, 2013.
Pursuant to the Assignment Agreement with Valent, the Company agreed to issue warrants to Valent under certain circumstances. The financing completed by the Company that closed in February 2012 constituted a Financing Transaction under the terms of the Assignment Agreement and resulted in the Company issuing 500,000 warrants to Valent on February 1, 2012 at an exercise price of CDN $0.50 per warrant (note 8). In exchange for the warrants Valent has assigned all of its right, title and interest in and to the patents for VAL-083 to the Company. The fair value of the contingent warrants of $89,432 has been recognized as an expense and a corresponding increase to additional paid-in capital at December 31, 2011. As a result of the warrants being issued during 2012 the amount previously recognized as additional paid in capital has been reclassified to warrants during the year ended December 31, 2012.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
|
5
|
Taxes and other receivables
|
| |
|
$ |
2013 |
|
|
$ |
2012 |
|
| |
|
|
|
|
|
|
|
|
|
Government grants
|
|
|
- |
|
|
|
34,168 |
|
|
Other receivables
|
|
|
11,062 |
|
|
|
11,331 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
11,062 |
|
|
|
45,499 |
|
On May 1, 2012 the Company was granted a non-repayable financial contribution of up to $48,245 (CDN $48,000) from the National Research Council of Canada Industrial Research Assistance Program (“IRAP”). The Company will be reimbursed for certain research and development costs to a maximum of $48,245 (CDN $48,000) in the period from May 1, 2012 thru November 30, 2012. Under this IRAP grant the Company requested an aggregate total reimbursement of $40,542 and has received $6,374 to December 31, 2012 resulting in a receivable of $34,168 at December 31, 2012. Under this IRAP grant the Company did not incur all of the allowable expenses under the grant and as a result $7,703 has lapsed.
Total amounts credited in the statement operations for all IRAP grants in 2013 was $nil (2012 - $40,542; 2011 - $66,724).
|
6
|
Accounts payable and accrued liabilities
|
| |
|
$ |
2013 |
|
|
$ |
2012 |
|
| |
|
|
|
|
|
|
|
|
|
Trade payables
|
|
|
140,457 |
|
|
|
677,615 |
|
|
Payable to related parties (note 9)
|
|
|
109,030 |
|
|
|
447,777 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
249,487 |
|
|
|
1,125,392 |
|
During the year ended December 31, 2012, the Company issued 500,000 common shares valued at $253,050 (CDN $250,000) as partial settlement of the Company’s accounts payable balance with Valent (note 8). The fair value of the shares issued as partial settlement was based on the financing which occurred during the year ended December 31, 2012.
The Company has issued stock purchase warrants. Based on the terms of certain of these warrants the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value each reporting period with the changes in fair value recorded in the consolidated statement of loss and comprehensive loss.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
CDN $0.50 Unit Warrants
The Company issued 4,150,000 units on January 23, 2012, 560,000 on February 27, 2012 and 50,000 on May 10, 2012. In addition, during the year ended December 31, 2011 the Company issued 500,000 units on October 3, 2011, 100,000 on October 7, 2011, and 50,000 on November 11, 2011. In total, the Company issued 5,410,000 units for services in settlement of accounts payable and cash proceeds for an aggregate of $2,671,923 (CDN $2,705,000).
The proceeds from the issuance of 3,000,000 of these units were held in escrow pursuant to an exclusive option investment agreement with a strategic investor. Subsequently, the Company elected to allow the option to expire and the related units were cancelled and the funds returned from escrow to the subscriber in order for the Company to retain control over certain intellectual property and commercial rights.
During the year ended December 31, 2013, 221,000 warrants were exercised for no additional consideration for 221,000 shares of common stock. As a result, $241,715 of the derivative liability has been reclassified to equity. The warrants that have been exercised were revalued at their exercise date and then the reclassification to equity was recorded.
Subsequent to December 31, 2013, 20,000 CDN $0.50 warrants were exercised for no additional consideration. In addition, on January 25, 2014 2,169,000 CDN $0.50 warrants expired. All of the CDN $0.50 warrants outstanding at December 31, 2013 have now either been exercised or have expired.
The remaining warrants issued with the units have been re-valued at December 31, 2013 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility – 72.8%, risk free rate - 0.09% and a term of one month.
Investor Warrants
In connection with the Reverse Acquisition (note 3), on January 25, 2013, January 31, 2013, February 8, 2013, February 21, 2013, February 28, 2013, March 1, 2013, and March 6, 2013, the Company entered into and closed a series of subscription agreements with accredited investors (the “Investors”), pursuant to which the Company issued an aggregate of 13,125,002 Units at a purchase price of $0.80 per Unit, for aggregate gross proceeds of $10,500,000 (the “Private Offering”). Each Unit consists of one share of common stock and one five-year warrant (the “Investor Warrants”) to purchase one share of common stock at an exercise price of $0.80. The exercise price of the Investor Warrants is subject to adjustment in the event that the Company sells common stock at a price lower than the exercise price, subject to certain exceptions. The Investor Warrants are redeemable by the Company at a price of $0.001 per Investor Warrant at any time subject to the conditions that (i) the Company’s common stock has traded for twenty (20) consecutive trading days with a closing price of at least $1.60 per share with an average trading volume of 50,000 shares per day and (ii) the underlying shares of common stock are registered.
The Investor Warrants issued with the units have been re-valued at December 31, 2013 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility - 78%, risk free rate - 1.3% and a term of approximately 4.25 years.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
Dividend Warrants
As a result of the Reverse Acquisition, warrants that Berry issued pursuant a warrant dividend became warrants of the Company (the “Dividend Warrants”). The Dividend Warrants are exercisable at $1.25 per share until January 24, 2018. The Dividend Warrants will only be exercisable at such times as the underlying shares of common stock are registered. The Dividend Warrants will be redeemable by the Company at a price of $0.001 per Dividend Warrant at any time commencing 18 months following the date of issuance subject to the conditions that (i) the Company’s common stock has traded for twenty (20) consecutive trading days with a closing price of at least $2.50 per share and (ii) the underlying shares of common stock are registered. Subject to the conditions set forth therein, the Dividend Warrants may be redeemed by the Company upon not less than ninety (60) days nor more than ninety (90) days prior written notice.
The Dividend Warrants have been measured at fair value at December 31, 2013 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility - 78%, risk free rate - 1.3% and a term of approximately 4 years.
Warrants issued for services
During the year ended December 31, 2013 the Company issued 300,000 warrants for services. The warrants were issued on September 12, 2013 and are exercisable on a cashless basis at an exercise price of $1.76 for five years. The Company has recognized $124,020 in expense in the consolidated statement of operations.
The warrants have been measured at fair value at their issue date of December 31, 2013 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility -88%, risk free rate - 1.8% and a term of approximately 4.75 years.
The Company’s derivative liability is summarized as follows:
| |
|
December 31,
2013
$
|
|
|
December 31,
2012
$
|
|
| |
|
|
|
|
|
|
|
Opening balance
|
|
|
121,000 |
|
|
|
106,146 |
|
| |
|
|
|
|
|
|
|
|
|
Issuance of units
|
|
|
3,681,372 |
|
|
|
333,356 |
|
|
Dividend warrant liability acquired on reverse acquisition
|
|
|
2,041,680 |
|
|
|
- |
|
|
Warrants issued for services
|
|
|
124,020 |
|
|
|
- |
|
|
Change in fair value of unexercised warrants
|
|
|
(1,324,051 |
) |
|
|
(318,502 |
) |
|
Reclassification to equity upon exercise of warrants
|
|
|
(241,715 |
) |
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
Closing balance
|
|
|
4,402,306 |
|
|
|
121,000 |
|
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
|
8
|
Stockholders’ deficiency
|
Preferred stock
Authorized
5,000,000 preferred shares, $0.001 par value
Issued and outstanding at December 31, 2013 - 1 (December 31, 2012 - none)
In connection with the Exchange Agreement (note 3), on the Closing Date, the Company, Callco, Exchangeco and Computershare Trust Company of Canada (the “Trustee”) entered into a voting and exchange trust agreement (the “Trust Agreement”). Pursuant to the Trust Agreement, Company issued one share of Special Voting Preferred Stock (the “Special Voting Share”) to the Trustee, and the parties created a trust for the Trustee to hold the Special Voting Share for the benefit of the holders of the Exchangeable Shares (other than the Company and any affiliated companies) (the “Beneficiaries”). Pursuant to the Trust Agreement, the Beneficiaries will have voting rights in the Company equivalent to what they would have had they received shares of common stock in the same amount as the Exchangeable Shares held by the Beneficiaries.
In connection with the Exchange Agreement and the Trust Agreement, on January 17, 2013, the Company filed a certificate of designation of Special Voting Preferred Stock (the “Special Voting Certificate of Designation”) with the Secretary of State of Nevada. Pursuant to the Special Voting Certificate of Designation, one share of the Company’s blank check preferred stock was designated as Special Voting Preferred Stock. The Special Voting Preferred Stock votes as a single class with the common stock and is entitled to a number of votes equal to the number of Exchangeable Shares of Exchangeco outstanding as of the applicable record date (i) that are not owned by the Company or any affiliated companies and (ii) as to which the holder has received voting instructions from the holders of such Exchangeable Shares in accordance with the Trust Agreement.
The Special Voting Preferred Stock is not entitled to receive any dividends or to receive any assets of the Company upon any liquidation, and is not convertible into common stock of the Company.
The voting rights of the Special Voting Preferred Stock will terminate pursuant to and in accordance with the Trust Agreement. The Special Voting Preferred Stock will be automatically cancelled at such time as the share of Special Voting Preferred Stock has no votes attached to it.
Common stock
Authorized
200,000,000 common shares, $0.001 par value
Issued and outstanding at December 31, 2013 - 31,534,819 (December 31, 2012 - 13,050,000). The issued and outstanding common shares include 7,374,583 shares of common stock on an as-exchanged basis with respect to the Exchangeable Shares (note 3).
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
|
a)
|
Shares issued to founders
|
On May 27, 2010, the Company issued 7,000,000 common shares to its founders at $0.001 per share for total proceeds of $6,667. Of the 7,000,000 shares issued, 6,000,000 were issued to founders who are also officers or directors of the Company. In addition, of the 7,000,000 shares issued, 6,700,000 are subject to vesting provisions and a repurchase option to the Company. At any time prior to the expiration of 36 months from May 27, 2010 the Company at its sole discretion may repurchase some or all of the unvested 6,700,000 shares at $0.001 per share.
With respect to the 6,700,000 shares subject to vesting, 25% of the common shares vested immediately on May 27, 2010 and the remaining shares shall vest in twelve equal tranches on each quarterly anniversary of May 27, 2010 with the number of shares to vest on each such date to equal 1/16 of the number of shares issued on May 27, 2010. If any of the subscribers is or becomes a director, officer, employee or consultant of the Company or an affiliate of the Company, all unvested shares shall vest immediately if the subscriber is subsequently removed as a director or officer of the Company or its affiliate, or is subsequently terminated as an employee or consultant of the Company or its affiliate, in each case without cause.
|
b)
|
Shares issued to the DelMar Employees Share Purchase Trust
|
The Company has established the DelMar Employees Share Purchase Trust (the “Trust”). The purposes of the Trust are to (i) enhance the ability of the Company and its affiliates to attract, motivate, retain and reward directors, officers, employees and consultants, (b) facilitate employee ownership of shares of the company and (c) promote closer alignment of interests between key employees of the company and its shareholders. The Trust is overseen by a Trustee appointed by the Company and funds from the Company (“Settled Funds”) were used to subscribe for common shares (“Trust Shares”) in the capital of the Company. On May 27, 2010, the Company issued 2,000,000 common shares to the trust. The Company used Settled Funds to pay for the trust Shares.
| |
|
Number of shares held in Trust
|
|
| |
|
|
|
|
Balance - April 6, 2010
|
|
|
- |
|
|
Shares issued to the DelMar Employee Share Purchase Trust
|
|
|
2,000,000 |
|
|
Shares transferred to employees and consultants for services
|
|
|
(325,000 |
) |
|
Founders shares acquired by the Trust
|
|
|
68,750 |
|
| |
|
|
|
|
|
Balance - December 31, 2010
|
|
|
1,743,750 |
|
|
Shares transferred to employees and consultants for services
|
|
|
(200,000 |
) |
|
Founders shares acquired by the Trust
|
|
|
46,875 |
|
| |
|
|
|
|
|
Balance - December 31, 2011
|
|
|
1,590,625 |
|
|
Shares transferred to employees and consultants for services
|
|
|
(1,590,625 |
) |
| |
|
|
|
|
|
Balance - December 31, 2013 and 2012
|
|
|
- |
|
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
The Company has transferred shares from the Trust to various consultants for work or services performed for the Company. Shares held by the Trust are not issued and outstanding until the shares are transferred out of the Trust. For the year ended December 31, 2012, the Company recognized the fair value of the shares transferred as an expense with the offsetting charge to capital stock for $781,846 (2011- $95,140, 2010 - $32,091). The Company did not recognize any expenses related to Trust shares for the year ended December 31, 2013 as all shares have been issued from the Trust as of December 31, 2012.
Of the 1,590,625 transferred out of the trust during the year ended December 31, 2012, 1,390,625 were transferred to directors of the Company. The related compensation expense was recorded in the consolidated statement of operations.
|
c)
|
Shares issued in private placements
|
On August 27, 2010, the Company issued 720,000 common shares at $0.095 (CDN $0.10) per share for total proceeds of $68,414 and on September 8, 2010 the Company issued an additional 280,000 common shares at $0.096 (CDN $0.10) per share for total proceeds of $26,989. Of the total proceeds of $68,414 from the August 27, 2010 issuance, $28,506 was received in 2011 and has been recorded as subscriptions receivable at December 31, 2010.
|
d)
|
Shares issued to Valent for settlement of accounts payable
|
During the year ended December 31, 2012, the Company issued 500,000 common shares to Valent for partial settlement of accounts payable (notes 6 and 9).
|
e)
|
Shares issued for the Reverse Acquisition
|
On January 25, 2013, the Company entered into and closed an Exchange Agreement with DelMar (BC) (note 3). The Reverse Acquisition resulted in the Company acquiring DelMar (BC) by issuing a sufficient number of shares such that the shareholders of DelMar (BC) had a controlling interest in the Company subsequent to the completion of the Reverse Acquisition. At the time of the Reverse Acquisition, there were 13,070,000 common shares of DelMar (BC) and 3,250,007 shares of common stock of the Company issued and outstanding. All of the 13,070,000 shares of DelMar (BC) were acquired either directly or indirectly (through Exchangeco) by the Company resulting in DelMar (BC) becoming a wholly owned subsidiary of the Company.
As a result of the shareholders of DelMar (BC) having a controlling interest in the Company subsequent to the Reverse Acquisition, for accounting purposes the transaction constitutes a reverse recapitalization with DelMar (BC) being the accounting acquirer even though legally the Company is the acquirer. Therefore, for accounting purposes, the Company is shown to have issued 3,250,007 common shares for the Reverse Acquisition (note 3).
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
In connection with the Reverse Acquisition, on January 25, 2013, January 31, 2013, February 8, 2013, February 21, 2013, February 28, 2013, March 1, 2013, and March 6, 2013, the Company entered into and closed a series of subscription agreements with accredited investors (the “Investors”), pursuant to which the Company issued an aggregate of 13,125,002 Units at a purchase price of $0.80 per Unit, for aggregate gross proceeds of $10,500,000 (the “Private Offering”). Each Unit consists of one share of common stock and one five-year warrant (the “Investor Warrants”) to purchase one share of common stock at an exercise price of $0.80. The exercise price of the Investor Warrants is subject to adjustment and the Investor Warrants are redeemable under certain circumstances (note 7).
The Company retained Charles Vista, LLC (the “Placement Agent”) as the placement agent for the Private Offering. The Company paid the Placement Agent a cash fee of $1,050,000 (equal to 10% of the gross proceeds), a non-accountable expense allowance of $315,000 (equal to 3% of the gross proceeds), and a one-year consulting fee of $60,000. In addition, the Company incurred other unit issue and closings costs of approximately $500,000 resulting in net proceeds to the Company of $8,575,000. Certain of the additional closing costs are not eligible to be treated as share issue costs and as a result they have been expensed. Net unit proceeds per the consolidated statements of cash flows include gross unit proceeds less cash share issue costs attributable to the shares only. The portion of the unit issue costs attributable to the derivative liability has been expensed.
In addition, the Company issued to the Placement Agent five-year warrants (the “Placement Agent Warrants”) to purchase 5,250,000 shares of common stock (equal to 20% of the shares of common stock (i) included as part of the Units sold in the Private Offering and (ii) issuable upon exercise of the Investor Warrants) at an exercise price of $0.80, exercisable on a cash or cashless basis. Pursuant to the cashless exercise provision in the Placement Agent Warrants, if the warrants are exercised on a cashless basis, the number of shares the Company will issue to the holder will be dependent on the closing price of the common stock for the immediately preceding 20 trading days.
The Company will pay a warrant commission of 5% of the amount of funds raised by an agent upon the exercise of the Investor Warrants following such redemption.
In connection with the Private Offering, the Company entered into a registration rights agreement with the Investors, pursuant to which the Company agreed to file a registration statement (the “Registration Statement”) registering for resale all shares of common stock (a) included in the Units; and (b) issuable upon exercise of the Investor Warrants, no later than 90 days after the completion of the Private Offering (the “Filing Deadline”) and to use commercially reasonable efforts to cause the Registration Statement to become effective within 180 days of the Filing Deadline. The Company agreed to use commercially reasonably efforts to keep the Registration Statement effective while the Investor Warrants are outstanding.
Certain of the Private Offering costs were incurred by the Company prior to December 31, 2012. These costs of $90,771 were treated as issue costs during the year ended December 31, 2013.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
|
g)
|
Shares issued to Valent for future royalty reduction
|
Simultaneous with the Reverse Acquisition, the Company issued to Valent 1,150,000 shares of common stock in exchange for Valent reducing certain future royalties under its Assignment Agreement with the Company (note 4).
|
h)
|
Shared issued for services
|
Pursuant to a consulting agreement dated May 1, 2012 the Company issued 20,000 shares of common stock per month from June 1, 2012 to May 1, 2013 inclusive. Under this agreement the Company has issued a total of 100,000 shares of common stock during the year ended December 31, 2013 (2012 – 140,000). The shares have been valued using the fair value of the Company shares based on the purchase price under recent shares issuance by the Company or the closing price of the common stock on the date the shares for services were issued. A total of $142,557 in expense has been recognized for these shares for the year ended December 31, 2013 (2012 - $75,800).
In addition to the shares issued under the May 1, 2012 consulting agreement, during the year ended December 31, 2013 the Company also issued 515,000 shares of common stock for services resulting in the recognition of $901,000 in expense for a total of shares for services expense of $1,043,557 (2012 - $75,800).
The total expense of $1,043,557 in addition to the stock option expense of $1,103,209 results in a total share-based payment expense of $2,146,766 for the year ended December 31, 2013 (2012 - $1,130,240). This total expense has been recognized as to $568,725 and $1,578,041 for research and development, and general and administrative respectively for the year ended December 31, 2013. For the year ended December 31, 2012 the total share-based payment expense of $1,130,240 has been recognized as to $746,356 and $383,884 for research and development, and general and administrative respectively.
Stock options
On February 1, 2012, the Company’s board of directors approved its stock option plan (the “Plan”). Under the Plan the number of common shares that will be reserved for issuance to officers, directors, employees and consultants under the Plan will not exceed 7.5% of the share capital of the Company on a fully diluted basis. The requisite service period of the options ranges from six months to three years and also have a range of six months to three years contractual term.
In the event of the sale of 66 2/3% of the equity securities of the Company where equity securities include shares, warrants, stock options, and any convertible securities of the Company, any options not yet granted under the Plan shall be deemed granted to the principle founders of the Company on a pro-rata basis in accordance with their ownership of the Company on a fully-diluted basis immediately prior to the closing of such a sale.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
The following table sets forth the options outstanding under the Plan as of December 31, 2013:
| |
|
Number of
stock
options
outstanding
|
|
|
Weighted
average
exercise
price
|
|
| |
|
|
|
|
|
|
|
Balance - December 31, 2011
|
|
|
- |
|
|
|
- |
|
|
Granted
|
|
|
1,020,000 |
|
|
|
0.47 |
|
| |
|
|
|
|
|
|
|
|
|
Balance – December 31, 2012
|
|
|
1,020,000 |
|
|
|
0.47 |
|
|
Granted
|
|
|
2,340,000 |
|
|
|
1.15 |
|
|
Cancelled
|
|
|
(120,000 |
) |
|
|
0.47 |
|
| |
|
|
|
|
|
|
|
|
|
Balance – December 31, 2013
|
|
|
3,240,000 |
|
|
|
0.96 |
|
| |
|
|
|
|
|
|
|
|
The following table summarizes stock options currently outstanding and exercisable at December 31, 2013:
|
Exercise price
$
|
|
|
Number
outstanding at
December 31,
2013
|
|
|
Weighted
average
remaining
contractual
life
(years)
|
|
|
Weighted
average
exercise
price
$
|
|
|
Number
exercisable
at
December 31,
2013
|
|
|
Exercise
price
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
0.47 |
|
|
|
900,000 |
|
|
|
8.08 |
|
|
|
0.47 |
|
|
|
726,333 |
|
|
|
0.47 |
|
| |
1.05 |
|
|
|
2,040,000 |
|
|
|
9.62 |
|
|
|
1.05 |
|
|
|
584,296 |
|
|
|
1.05 |
|
| |
1.54 |
|
|
|
180,000 |
|
|
|
9.25 |
|
|
|
1.54 |
|
|
|
180,000 |
|
|
|
1.54 |
|
| |
2.30 |
|
|
|
120,000 |
|
|
|
9.42 |
|
|
|
2.30 |
|
|
|
70,000 |
|
|
|
2.30 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
3,240,000 |
|
|
|
|
|
|
|
0.96 |
|
|
|
1,560,629 |
|
|
|
0.89 |
|
Included in the number of stock options outstanding are 900,000 stock options granted at an exercise price of CDN $0.50. The exercise prices shown in the above table have been converted to USD using the period ending closing exchange rate resulting in an exercise price of $0.47. Certain stock options have been granted to non-employees and will be revalued at each reporting date until they have fully vested. The stock options have been valued using a Black-Scholes pricing model using the following assumptions:
| |
|
December 31,
2013
$
|
|
|
December 31,
2012
$
|
|
| |
|
|
|
|
|
|
|
Dividend rate
|
|
|
0 |
% |
|
|
0 |
% |
|
Volatility
|
|
73% to 85%
|
|
|
|
74 |
% |
|
Risk-free rate
|
|
|
1.00 |
% |
|
|
1.25 |
% |
|
Term - years
|
|
1 to 3
|
|
|
|
2.1 |
|
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
The Company has recognized the following amounts as stock-based compensation expense for the periods noted:
| |
|
Periods ended December 31,
|
|
| |
|
|
|
|
|
|
| |
|
$ |
2013 |
|
|
$ |
2012 |
|
| |
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
522,725 |
|
|
|
196,281 |
|
|
General and administrative
|
|
|
580,484 |
|
|
|
76,313 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
1,103,209 |
|
|
|
272,594 |
|
Of the total stock option expense of $1,103,209, $890,648 (2012 - $272,594; 2011 - $nil) has been recognized as additional paid in capital and $212,561 (2012 - $nil; 2011 - $nil) has been recognized as a stock option liability.
The aggregate intrinsic value of stock options outstanding at December 31, 2013 was $422,910 (2012 - $306,000; 2011 - $nil) and the aggregate intrinsic value of stock options exercisable at December 31, 2013 was $341,304 (2012 - $172,650; 2011 - $nil). As of December 31, 2013 there was $456,301 in unrecognized compensation expense that will be recognized over the next 2.5 years. No stock options granted under the Plan have been exercised to December 31, 2013. Upon the exercise of stock options new shares will be issued.
A summary of status of the Company’s unvested stock options as of December 31, 2013 under all plans is presented below:
| |
|
Number of
options
|
|
|
Weighted
average
exercise
price
$
|
|
|
Weighted
average
grant date
fair value
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
Unvested at December 31, 2011
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Granted
|
|
|
1,020,000 |
|
|
|
0.47 |
|
|
|
0.30 |
|
|
Vested
|
|
|
(575,500 |
) |
|
|
0.47 |
|
|
|
0.30 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unvested at December 31, 2012
|
|
|
444,500 |
|
|
|
0.47 |
|
|
|
0.30 |
|
|
Granted
|
|
|
2,340,000 |
|
|
|
1.15 |
|
|
|
0.63 |
|
|
Cancelled
|
|
|
(120,000 |
) |
|
|
0.47 |
|
|
|
0.30 |
|
|
Vested
|
|
|
(985,129 |
) |
|
|
1.05 |
|
|
|
0.58 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unvested at December 31, 2013
|
|
|
1,679,371 |
|
|
|
1.08 |
|
|
|
0.59 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
The aggregate intrinsic value of unvested stock options at December 31, 2013 was $81,606 (2012 - $133,350; 2011 - $nil). The unvested stock options have a remaining weighted average contractual term of 9.46 years.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
Warrants
| |
|
Number of
warrants
|
|
|
Amount
$
|
|
| |
|
|
|
|
|
|
|
Balance - December 31, 2011
|
|
|
- |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
Warrants issued for patents (i)
|
|
|
500,000 |
|
|
|
89,432 |
|
|
Warrants issued as unit issue costs (ii)
|
|
|
105,000 |
|
|
|
14,295 |
|
|
Warrants issued for services (iii)
|
|
|
345,000 |
|
|
|
49,379 |
|
| |
|
|
|
|
|
|
|
|
|
Balance - December 31, 2012
|
|
|
950,000 |
|
|
|
153,106 |
|
|
Warrants issued as unit issue costs (iv)
|
|
|
5,250,000 |
|
|
|
6,288,594 |
|
|
Warrants exercised on a cashless basis (v)
|
|
|
(200,000 |
) |
|
|
(239,600 |
) |
| |
|
|
|
|
|
|
|
|
|
Balance - December 31, 2013
|
|
|
6,000,000 |
|
|
|
6,202,100 |
|
|
i)
|
At December 31, 2011, the Company recognized the fair value of the 500,000 contingent Valent warrants (note 4). The contingent warrants were recognized in additional paid in capital at December 31, 2011 and have been reclassified to warrants when the warrants were issued on February 1, 2012. The warrants have an exercise price of CDN $0.50 per warrant and expire February 1, 2017.
|
|
ii)
|
The Company has issued broker warrants as finder’s fees in relation to the issuance of certain units. All of the warrants were issued on March 1, 2012 and have an exercise price of CDN $0.50 per warrant. Of the total, 100,000 expire March 1, 2015 and 5,000 expire March 1, 2014.
|
|
iii)
|
The Company has issued 345,000 warrants for investor relations services. The warrants were issued on February 1, 2012 and they vest in 12 equal installments over a 12-month period commencing on March 1, 2012. The warrants have an exercise price of CDN $0.50 per warrant and expire February 1, 2015.
|
|
iv)
|
As part of the Company’s unit offering the Company has issued 5,250,000 Placement Agent Warrants (note 8(f)). The Placement Agent Warrants have been recognized as non-cash issue costs and the costs have been allocated to common stock and derivative liability. The portion allocated to additional paid in capital was $4,087,586 and the portion allocated to derivative liability was $2,201,008. The Placement Agent warrants have been valued using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility - 104%, risk free rate - 1.0% and a term of five years.
|
|
v)
|
During the year ended December 31, 2013 200,000 Placement Agent Warrants were exercised on a cashless basis for 123,810 shares of common stock.
|
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
The fair value of all of the warrants issued in 2012 and 2011 was based on the fair value of the warrants included as part of the unit issuances completed in 2011 and 2012. The fair value of the warrants issued in 2013 was determined by independent valuation as part of the valuation performed for the Company’s derivative liability (note 7).
Certain of the Company’s warrants have been recognized as a derivative liability (note 7).
The following table summarizes all of the Company’s outstanding warrants as of December 31, 2013:
|
Description
|
|
Number
|
|
| |
|
|
|
|
CDN $0.50 warrants (note 7) (i)
|
|
|
2,189,000 |
|
|
Issued as broker warrants (ii)
|
|
|
105,000 |
|
|
Issued for patents (iii)
|
|
|
500,000 |
|
|
Issued for services (iv)
|
|
|
345,000 |
|
|
Investor Warrants (note 7) (v)
|
|
|
13,125,002 |
|
|
Dividend warrants (note 7)(vi)
|
|
|
3,250,007 |
|
|
Placement Agent (note 8(f))(vii)
|
|
|
5,050,000 |
|
|
Issued for services (viii)
|
|
|
300,000 |
|
| |
|
|
|
|
|
Closing balance - December 31, 2013
|
|
|
24,864,009 |
|
|
i)
|
All of the warrants expire on January 25, 2014. They are exercisable at $1.20 per warrant until that date. A total of 20,000 warrants are exercisable for no additional consideration. Subsequent to December 31, 2013 the 20,000 warrants were exercised for no additional consideration and the remaining 2,169,000 expired (note 13).
|
|
ii)
|
The Company has issued broker warrants as finder’s fees in relation to the issuance of certain of the CDN $0.50 units issued during the years ended December 31, 2011 and 2012. All of the warrants were issued on March 1, 2012 and have an exercise price of CDN $0.50 per warrant. Of the total, 100,000 expire March 1, 2015 and 5,000 expire March 1, 2014. On March 1, 2014, 5,000 warrants expired (note 13).
|
|
iii)
|
The Company issued 500,000 warrants to Valent (note 4). The warrants have an exercise price of CDN $0.50 per warrant and expire February 1, 2017.
|
|
iv)
|
The Company has issued 345,000 warrants for investor relations services. The warrants were issued on February 1, 2012 and they vested in 12 equal installments over a 12-month period commencing on March 1, 2012. The warrants have an exercise price of CDN $0.50 per warrant and expire February 1, 2015.
|
|
v)
|
The Investor Warrants were issued as part of the Company’s $0.80 unit offering. They were issued in tranches on January 25, 2013, January 31, 2013, February 8, 2013, February 21, 2013, February 28, 2013, March 1, 2013, and March 6, 2013 respectively (note 8(f)). They are exercisable at $0.80 per warrant for five years commencing from their respective issue dates.
|
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
|
vi)
|
The Dividend Warrants are exercisable at $1.25 per warrant until January 24, 2018.
|
|
vii)
|
The Placement Agent Warrants are exercisable at $0.80 per warrant until March 6, 2018 but can be exercised on a cashless basis. The Placement Agent Warrants were all issued on March 6, 2013.
|
|
viii)
|
The warrants are exercisable on a cashless basis at a price of $1.76 per warrant until September 12, 2018.
|
|
9
|
Related party transactions
|
During the year ended December 31, 2013
The Company paid total cash compensation to its officers of $454,549 for the twelve months ended December 31, 2013.
Included in accounts payable at December 31, 2013 is an aggregate amount owing of $74,754 to the Company’s officers and directors for fees and expenses. The Company pays related party payables incurred for fees and expenses under normal commercial terms.
Included in related party payables at December 31, 2013 is an amount of $44,007 relating to clinical development costs incurred by Valent on behalf of the Company. Additionally, the Company also has a loan payable of $272,372, including accrued interest of $22,372, due to Valent (note 4). One of the directors and officers of the Company is also a Principal of Valent. As a result of the Company not expecting to repay Valent within the next twelve months, the balance of the loan and accrued interest has been disclosed as a long-term liability.
On January 25, 2013, in connection with the Reverse Acquisition (note 3), Valent was issued 1,150,000 shares of common stock of the Company in exchange for Valent reducing certain future royalties under the Assignment Agreement (note 8(g)). As a result of the share issuance the Company has recognized an expense of $598,000 for the year ended December 31, 2013.
The Company granted an aggregate 1,410,000 stock options at an exercise price of $1.05 to its officers and directors (note 8).
The Company recognized $44,333 in directors’ fees.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
During the year ended December 31, 2012
Pursuant to consulting agreements with the Company’s officers and directors the Company paid a total of $27,022 (CDN $27,000) per month to its officers and directors during the year. Under two of these agreements the directors have elected to receive a portion of their aggregate compensation in the form of units. The Company issued 360,000 units for a total amount of $180,144. The units issued relate to an amount of $15,012 (CDN $15,000) per month from January to December 2012 inclusive. All of the units were issued in February 2012. The Company has recognized $180,144 in services for the year ended December 31, 2012. Of the $180,144, $60,389 has been recognized as general and administrative and $119,755 has been recognized as research and development.
Additionally, under the consulting agreements the Company has paid its officers and directors cash compensation totaling an aggregate $12,006 (CDN $12,000) per month. An amount of $144,072 (CDN $144,000) has been paid in cash to the two individuals for the year ended December 31, 2012.
Included in related party payables at December 31, 2012 is an aggregate amount owing of $133,658 to the Company’s directors in relation to their respective consulting agreements and for reimbursable expenses.
Also included in related party payables December 31, 2012 is an amount of $314,119 relating to clinical development costs incurred by Valent on behalf of the Company. On April 30, 2012, Valent was issued 500,000 common shares for partial settlement of the Company’s accounts payable balance with Valent. The total settlement amount was $253,050. Additionally, the Company has a loan payable, including accrued interest, of $264,352 due to Valent (note 4).
Through a Company owned by one of the Company’s directors, a $25,000 retainer was paid pursuant to the unit financing completed by the Company (note 8). The $25,000 is included in accounts payable at December 31, 2012.
The Company granted an aggregate 450,000 stock options at an exercise price of $0.47 (CDN $0.50) to its directors (note 8).
The Company transferred a total of 1,390,625 shares from the DelMar Employee Share Purchase Trust to the Company’s directors (note 8).
During the year ended December 31, 2011
Pursuant to consulting agreements dated August 1, 2011 with the Company’s officers and directors the Company agreed to compensate its officers and directors for services rendered to the Company. An aggregate $26,550 (CDN $27,000) per month commencing August 1, 2011 and ending December 31, 2012 will be payable pursuant the consulting agreements. Under the consulting agreements the Company and the respective officer or director have mutually agreed that a portion of the compensation payable under the respective agreement shall be deemed to have been invested in the unit offering of the Company as of October 3, 2011. The units issued under these agreements shall have the same terms as the CDN $0.50 units issued by the Company to subscribers of the offering (note 7).
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
For the period from August 1 to December 31, 2011 $19,028 (CDN $20,000) per month was settled by the Company with units resulting in 150,000 units being issued. Total research and development expenses of $71,355 (CDN $75,000) and general and administrative expenses of $23,785 (CDN $25,000) have been recorded for this issuance of units.
The Company also issued 50,000 units to one of its officers for the settlement of accounts payable in the amount of $23,785 (CDN $25,000). The units were measured at fair value using the valuation estimate consistent with the most recent financing.
Included in related party payables at December 31, 2011 is an aggregate amount owing of $21,028 to two of the Company’s directors.
Also included in related party payable at December 31, 2011 is an amount of $496,932 relating to clinical development costs incurred by Valent on behalf of the Company. The Company also has a loan payable, including accrued interest, of $256,831 due to Valent at December 31, 2011.
|
10
|
Current and future income taxes
|
The Company has the following non-capital losses available to reduce taxable income of future years:
|
Expiry date
|
|
$ |
|
| |
|
|
|
|
2029
|
|
|
65,242 |
|
|
2030
|
|
|
1,102,400 |
|
|
2031
|
|
|
1,159,614 |
|
|
2033
|
|
|
4,275,931 |
|
Significant components of the Company’s future tax assets are shown below:
| |
|
|
2013
$
|
|
|
|
2012
$
|
|
| |
|
|
|
|
|
|
|
|
|
Non-capital losses carried forward
|
|
|
1,822,341 |
|
|
|
323,910 |
|
|
Financing costs
|
|
|
4,115 |
|
|
|
4,302 |
|
|
Scientific research and development
|
|
|
121,490 |
|
|
|
11,193 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
1,947,946 |
|
|
|
339,405 |
|
|
Valuation allowance
|
|
|
(1,947,946 |
) |
|
|
(339,405 |
) |
| |
|
|
|
|
|
|
|
|
|
Net future tax assets
|
|
|
- |
|
|
|
- |
|
The income tax benefit of these tax attributes have not been recorded in these financial statements because of the uncertainty of their recovery.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
The Company’s effective income tax rate differs from the statutory income tax rate of 34% (2012 - 13.5%, 2011 - 13.5%).
The differences arise from the following items:
| |
|
|
2013
$
|
|
|
|
2012
$
|
|
| |
|
|
|
|
|
|
|
|
|
Tax recovery at statutory income tax rates
|
|
|
(2,818,834 |
) |
|
|
(324,049 |
) |
|
Permanent differences
|
|
|
979,359 |
|
|
|
133,365 |
|
|
Effect of rate differentials between jurisdictions
|
|
|
320,965
|
|
|
|
- |
|
|
Other
|
|
|
- |
|
|
|
13,087 |
|
|
Effect of tax rate changes on future taxes
|
|
|
(305,647 |
) |
|
|
- |
|
|
Change in valuation allowance
|
|
|
1,824,157 |
|
|
|
177,597 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
- |
|
|
|
- |
|
|
11
|
Commitments and contingencies
|
Office Lease
The Company currently rents its offices pursuant to a one-year lease that commenced on November 1, 2013 at a rate of $2,185 (CDN$2,325) per month. During the year ended December 31, 2013, the Company recorded $22,323 as rent expense (2012 - $12,669; 2011 - $480).
|
12
|
Financial risk management
|
Market risk
Market risk is the risk that changes in market prices, such as foreign exchange rates, interest rates and equity prices will affect the Company’s income or valuation of its financial instruments.
The Company is exposed to financial risk related to fluctuation of foreign exchange rates. Foreign currency risk is limited to the portion of the Company’s business transactions denominated in currencies other than the United Sates dollar, primarily general and administrative expenses incurred in CDN dollars. The Company believes that the results of operations, financial position and cash flows would be affected by a sudden change in foreign exchange rates, but would not impair or enhance its ability to pay its CDN dollar accounts payable. The Company manages foreign exchange risk by converting its US to Canadian dollars as needed. The Company has only recently opened a US dollar bank account but maintains the majority of its cash in USD. As at December 31, 2013, Canadian dollar denominated accounts payable and accrued liabilities exposure in US dollars totaled $90,143.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
Foreign exchange risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rates. If foreign exchange rates were to fluctuate within +/-10% of the closing rate at year end the maximum exposure is $9,014.
Balances in foreign currencies at December 31, 2013 and 2012 are as follows:
| |
|
2013
CDN balances
$
|
|
|
2012
CDN balances
$
|
|
| |
|
|
|
|
|
|
|
Trade payables
|
|
|
95,835 |
|
|
|
359,088 |
|
|
Cash
|
|
|
75,474 |
|
|
|
17,873 |
|
The Company is subject to interest rate risk on its cash and cash equivalents and believes that the results of operations, financial position and cash flows would not be significantly affected by a sudden change in market interest rates relative to the investment interest rates due to the short-term nature of the investments. As at December 31, 2013, cash and cash equivalents held in Canadian dollar savings accounts or short-term investments of $70,961. The Company’s cash balance currently earns interest at standard bank rates. If interest rates were to fluctuate within +/-10% of the closing rate at year end the impact of the Company’s interest bearing accounts will be insignificant.
The only financial instruments that expose the Company to interest rate risk are its cash and cash equivalents.
Liquidity risk
Liquidity risk is the risk that the Company will encounter difficulty in raising funds to meet cash flow requirements associated with financial instruments. The recent problems in the global credit markets have resulted in a drastic reduction in the ability of companies to raise capital through the public markets. See note 1 going concern, for additional comments relating to liquidity risk. The Company continues to manage its liquidity risk based on the outflows experienced for the year ended December 31, 2013 and is undertaking efforts to conserve cash resources wherever possible. The maximum exposure of the Company’s liquidity risk is $521,859 at December 31, 2013 (2012 - $1,389,744).
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
(in US dollars unless otherwise noted)
Credit risk
Credit risk arises from cash and cash equivalents, deposits with banks and financial institutions, as well as outstanding receivables. The Company limits its exposure to credit risk, with respect to cash and cash equivalents, by placing them with high quality credit financial institutions. The Company’s cash equivalents consist primarily of operating funds with commercial banks. Of the amounts with financial institutions on deposit, the following table summarizes the amounts at risk should the financial institutions with which the deposits are held cease trading:
The maximum exposure of the Company’s credit risk is $11,062 at December 31, 2013 (2012 - $45,499).
|
Cash and
cash
equivalents
$
|
|
|
Insured
amount
$
|
|
|
Non-insured
amount
$
|
|
| |
|
|
|
|
|
|
|
| |
4,136,803 |
|
|
|
70,961 |
|
|
|
4,065,842 |
|
Concentration of credit risk
Financial instruments that subject the Company to credit risk consist primarily of cash and cash equivalents.
The Company places its cash and cash equivalents in accredited financial institutions and therefore the Company’s management believes these funds are subject to minimal credit risk. The Company has no significant off-balance sheet concentrations of credit risk such as foreign currency exchange contracts, option contracts or other hedging arrangements.
CDN $0.50 warrants
Subsequent to December 31, 2013, 20,000 CDN $0.50 warrants were exercised or no additional consideration. In addition, on January 25, 2014 2,169,000 CDN $0.50 warrants expired. All of the CDN $0.50 warrants outstanding at December 31, 2013 have now either been exercised or have expired.
Broker Warrants
On March 1, 2014, 5,000 broker warrants expired.
Investor warrants
Subsequent to December 31, 2013, 127,313 Investor Warrants were exercised for 127,313 shares of common stock at an exercise price of $0.80 per warrant for total proceeds of $101,850.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Consolidated Condensed Interim Balance Sheets
(expressed in US dollars unless otherwise noted)
| |
|
Note
|
|
|
March 31,
2014
$
|
|
|
December 31, 2013
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Current assets
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
|
|
|
|
3,474,150 |
|
|
|
4,136,803 |
|
|
Taxes and other receivables
|
|
|
|
|
|
10,419 |
|
|
|
11,062 |
|
|
Prepaid expenses
|
|
|
|
|
|
269,584 |
|
|
|
170,883 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
3,754,153 |
|
|
|
4,318,748 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities
|
|
|
|
|
|
404,413 |
|
|
|
140,457 |
|
|
Related party payables
|
|
|
3 |
|
|
|
35,798 |
|
|
|
109,030 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
440,211 |
|
|
|
249,487 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loan payable to Valent
|
|
|
|
|
|
|
274,387 |
|
|
|
272,372 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock option liability
|
|
|
5 |
|
|
|
241,863 |
|
|
|
212,561 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative liability
|
|
|
4 |
|
|
|
5,857,991 |
|
|
|
4,402,306 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
6,814,452 |
|
|
|
5,136,726 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Deficiency
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Authorized
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5,000,000 shares, $0.001 par value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 share outstanding at March 31, 2014
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(December 31, 2013 - 1)
|
|
|
5 |
|
|
|
- |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Authorized
|
|
|
|
|
|
|
|
|
|
|
|
|
|
200,000,000 shares, $0.001 par value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issued 32,082,132 at March 31 2014 (December 31, 2013 - 31,534,819)
|
|
|
5 |
|
|
|
32,082 |
|
|
|
31,535 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
5 |
|
|
|
9,748,010 |
|
|
|
8,791,715 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants
|
|
|
5 |
|
|
|
6,201,544 |
|
|
|
6,202,100 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Deficit accumulated during the development stage
|
|
|
|
|
|
|
(19,063,113 |
) |
|
|
(15,864,506 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income
|
|
|
|
|
|
|
21,178 |
|
|
|
21,178 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
(3,060,299 |
) |
|
|
(817,978 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
3,754,153 |
|
|
|
4,318,748 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Going concern and nature of operations (note 1)
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Subsequent events (note 7)
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated condensed interim financial statements.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Consolidated Condensed Interim Statement of Loss and Comprehensive Loss
(expressed in US dollars unless otherwise noted)
| |
|
|
|
|
Three months ended March 31,
|
|
|
Period from
April 6, 2010
(inception)
to March 31,
|
|
| |
|
Notes
|
|
|
|
2014
$
|
|
|
|
2013
$
|
|
|
|
2014
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
|
|
|
618,869 |
|
|
|
631,947 |
|
|
|
5,604,809 |
|
|
General and administrative
|
|
|
|
|
|
966,923 |
|
|
|
920,377 |
|
|
|
6,383,235 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
1,585,792 |
|
|
|
1,552,324 |
|
|
|
11,988,044 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other loss (income)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of derivative liability
|
|
|
4 |
|
|
|
1,599,349 |
|
|
|
2,543,574 |
|
|
|
(43,204 |
) |
|
Issuance of shares to Valent for future royalty reduction
|
|
|
|
|
|
|
- |
|
|
|
598,000 |
|
|
|
598,000 |
|
|
Derivative issue costs
|
|
|
|
|
|
|
- |
|
|
|
2,713,220 |
|
|
|
2,737,962 |
|
|
Foreign exchange loss (gain)
|
|
|
|
|
|
|
11,947 |
|
|
|
(3,754 |
) |
|
|
14,125 |
|
|
Interest expense
|
|
|
|
|
|
|
2,015 |
|
|
|
1,955 |
|
|
|
39,489 |
|
|
Interest income
|
|
|
|
|
|
|
(496 |
) |
|
|
- |
|
|
|
(2,987 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
1,612,815 |
|
|
|
5,852,995 |
|
|
|
3,343,385 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the period
|
|
|
|
|
|
|
3,198,607 |
|
|
|
7,405,319 |
|
|
|
15,331,429 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share
|
|
|
|
|
|
|
(0.10 |
) |
|
|
(0.30 |
) |
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares
|
|
|
|
|
|
|
31,659,791 |
|
|
|
24,316,325 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
|
|
|
|
3,198,607 |
|
|
|
7,405,319 |
|
|
|
15,331,429 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Translation to US dollar presentation currency
|
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
(21,178 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss
|
|
|
|
|
|
|
3,198,607 |
|
|
|
7,405,319 |
|
|
|
15,310,251 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated condensed interim financial statements.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Consolidated Condensed Interim Statement of Cash Flows
(expressed in US dollars unless otherwise noted)
| |
|
Three months ended March 31,
|
|
|
Period from
April 6, 2010
(inception) to
March 31,
|
|
| |
|
|
2014
$
|
|
|
|
2013
$
|
|
|
|
2014
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss for the period
|
|
|
(3,198,607 |
) |
|
|
(7,405,319 |
) |
|
|
(15,331,429 |
) |
|
Items not affecting cash
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accrued interest
|
|
|
2,015 |
|
|
|
1,955 |
|
|
|
24,387 |
|
|
Change in fair value of derivative liability
|
|
|
1,599,349 |
|
|
|
2,543,574 |
|
|
|
(43,204 |
) |
|
Shares issued to Valent for future royalty reduction
|
|
|
- |
|
|
|
598,000 |
|
|
|
598,000 |
|
|
Non-cash derivative issue costs
|
|
|
- |
|
|
|
2,201,008 |
|
|
|
2,201,008 |
|
|
Units issued for services
|
|
|
- |
|
|
|
- |
|
|
|
275,284 |
|
|
Warrants issued for patents
|
|
|
- |
|
|
|
- |
|
|
|
89,432 |
|
|
Warrants issued for services
|
|
|
- |
|
|
|
- |
|
|
|
173,399 |
|
|
Share-based compensation
|
|
|
620,074 |
|
|
|
272,902 |
|
|
|
4,024,311 |
|
|
Prototype drug product
|
|
|
- |
|
|
|
- |
|
|
|
250,000 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
(977,169 |
) |
|
|
(1,787,880 |
) |
|
|
(7,738,812 |
) |
|
Changes in non-cash working capital
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Taxes and other receivables
|
|
|
643 |
|
|
|
(31,395 |
) |
|
|
(10,419 |
) |
|
Prepaid expenses
|
|
|
(98,701 |
) |
|
|
(54,752 |
) |
|
|
(269,584 |
) |
|
Accounts payable and accrued liabilities
|
|
|
263,956 |
|
|
|
(98,722 |
) |
|
|
631,331 |
|
|
Related party payables
|
|
|
(73,232 |
) |
|
|
(151,718 |
) |
|
|
35,798 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
92,666 |
|
|
|
(336,587 |
) |
|
|
387,126 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
(884,503 |
) |
|
|
(2,124,467 |
) |
|
|
(7,351,686 |
) |
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net proceeds from the exercise of warrants
|
|
|
221,850 |
|
|
|
- |
|
|
|
221,850 |
|
|
Net proceeds from the issuance of units
|
|
|
- |
|
|
|
9,639,520 |
|
|
|
10,501,916 |
|
|
Net proceeds from the issuance of common shares
|
|
|
- |
|
|
|
- |
|
|
|
102,070 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
221,850 |
|
|
|
9,639,520 |
|
|
|
10,825,836 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase in cash and cash equivalents
|
|
|
(662,653 |
) |
|
|
7,515,053 |
|
|
|
3,474,150 |
|
|
Cash and cash equivalents - beginning of period
|
|
|
4,136,803 |
|
|
|
17,782 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents - end of period
|
|
|
3,474,150 |
|
|
|
7,532,835 |
|
|
|
3,474,150 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplementary information
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of shares for the settlement of accounts payable
|
|
|
- |
|
|
|
- |
|
|
|
253,050 |
|
|
Issuance of units for the settlement of accounts payable
|
|
|
- |
|
|
|
- |
|
|
|
23,785 |
|
|
Non-cash share issuance costs
|
|
|
- |
|
|
|
6,288,594 |
|
|
|
6,302,889 |
|
|
Settlement of accounts payable with a loan payable
|
|
|
- |
|
|
|
- |
|
|
|
250,000 |
|
|
Cashless exercise of Placement Agent Warrants
|
|
|
- |
|
|
|
- |
|
|
|
239,600 |
|
|
Exercise of CDN $0.50 Warrants for no additional consideration (note 4)
|
|
|
17,600 |
|
|
|
- |
|
|
|
259,315 |
|
|
Deferred costs
|
|
|
- |
|
|
|
90,771 |
|
|
|
- |
|
The accompanying notes are an integral part of these consolidated condensed interim financial statements.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
|
1
|
Going concern and nature of operations
|
Going concern
For the three months ended March 31, 2014, the Company reported a loss of $3,198,607 and an accumulated deficit of $19,063,113 at that date. As at March 31, 2014 the Company had cash and cash equivalents on hand of $3,474,150. The Company does not have the prospect of achieving revenues in the near future and the Company will require additional funding to maintain its research and development projects and for general operations. These circumstances lend substantial doubt as to the ability of the Company to meet its obligations as they come due. The expenses to be incurred in developing and pursuing our Company’s business plan have a large degree of uncertainty. In addition, the Company has not begun to commercialize or generate revenues from any product candidate.
Consequently, management is pursuing various financing alternatives to fund the Company’s operations so it can continue as a going concern in the medium to longer term. Accordingly, the Company is considered to be in the development stage as defined in Accounting Standards Codification (ASC) 915-10. We believe, based on our current estimates and plans that we have enough cash to fund our operations for the next 9 to 12 months. Management plans to secure the necessary financing through the issue of new equity and/or the entering into of strategic partnership arrangements. Nevertheless, there is no assurance that these initiatives will be successful.
These financial statements have been prepared on a going concern basis which assumes that the Company will continue its operations for the foreseeable future and contemplates the realization of assets and the settlement of liabilities in the normal course of business.
The conditions and risks noted above cast substantial doubt on the validity of that assumption. These financial statements do not give effect to any adjustments to the amounts and classification of assets and liabilities that may be necessary and could potentially be material, should the Company be unable to continue as a going concern.
Nature of operations
DelMar Pharmaceuticals, Inc. (the “Company”) is a Nevada corporation formed on June 24, 2009 under the name Berry Only Inc. On January 25, 2013 the Company acquired (either directly or indirectly) (the “Reverse Acquisition”) all of the issued and outstanding shares of DelMar Pharmaceuticals (BC) Ltd. (“DelMar BC”). Prior to the Reverse Acquisition, Berry did not have any significant assets or operations. DelMar Pharmaceuticals, Inc. is the parent company of DelMar (BC), a British Columbia, Canada corporation incorporated on April 6, 2010, which is a development stage company with a focus on the development of drugs for the treatment of cancer. It is also the parent company to 0959454 B.C. Ltd., a British Columbia corporation (“Callco”), and 0959456 B.C. Ltd., a British Columbia corporation (“Exchangeco”). Callco and Exchangeco were formed to facilitate the Reverse Acquisition.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
As a result of the shareholders of DelMar (BC) having a controlling interest in the Company subsequent to the Reverse Acquisition, for accounting purposes the transaction is a capital transaction with DelMar (BC) being the accounting acquirer even though the legal acquirer is Berry. Therefore, the historic financial statements of DelMar (BC) are presented as the comparative balances for the periods prior to the Reverse Acquisition.
References to the Company refer to the Company and its wholly-owned subsidiaries, DelMar (BC), Callco and Exchangeco. References to Berry relate to the Company prior to the Reverse Acquisition.
The Company is a development stage company focused on the discovery and development of new medicines with the potential to treat cancer patients who have failed modern targeted or biologic therapy. The Company has initiated a clinical trial with its lead drug candidate VAL-083 for the treatment of refractory glioblastoma multiforme (“GBM”). The Phase I/II study is an open-label, single arm dose-escalation study designed to evaluate the safety, tolerability, pharmacokinetics and anti-tumor activity of VAL-083 in patients with histologically confirmed initial diagnosis of primary WHO Grade IV malignant glioma, now recurrent. Patients with prior low-grade glioma or anaplastic glioma are eligible if histologic assessment demonstrates transformation to GBM.
The address of the Company’s administrative offices is Suite 720 - 999 West Broadway, Vancouver, British Columbia, V5Z 1K5 with clinical operations located at 3485 Edison Way, Suite R, Menlo Park, California, 94025.
|
2
|
Significant accounting policies
|
Basis of presentation
The consolidated condensed interim financial statements of the Company have been prepared in accordance with United States Generally Accepted Accounting Principles (“US GAAP”) and are presented in United States dollars. The Company’s functional currency is the United States dollar.
In the quarter ended March 31, 2013, the Company’s functional currency changed from Canadian dollars to United States dollars as a result of various objective factors. Therefore translation of goods and services in a foreign currency are re-measured to the functional currency of the Company with gains and losses on re-measurement recorded in the consolidated condensed interim statement of loss. Any gains and losses that were previously recorded in accumulated other comprehensive income are unchanged from the date of the change of functional currency which was January 1, 2013.
The accompanying consolidated condensed interim financial statements include the accounts of the Company and its wholly-owned subsidiaries, DelMar BC, Callco, and Exchangeco. All intercompany balances and transactions have been eliminated.
The principal accounting policies applied in the preparation of these financial statements are set out below and have been consistently applied to all periods presented.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
Unaudited interim financial data
The accompanying unaudited March 31, 2014 consolidated condensed interim balance sheets, the consolidated condensed interim statements of loss and comprehensive loss for the three months ended March 31, 2014 and 2013, and consolidated condensed cash flows for the three months ended March 31, 2014 and 2013, and the related interim information contained within the notes to the consolidated condensed interim financial statements have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission for interim financial information. Accordingly, they do not include all of the information and the notes required by accounting principles generally accepted in the United States for complete financial statements. These consolidated condensed interim financial statements should read in conjunction with the annual financial statements of the Company as at December 31, 2013 filed in our Form 10-K filed with the Securities and Exchange Commission on March 10, 2014. In the opinion of management, the unaudited consolidated condensed interim financial statements reflect all adjustments, consisting of normal and recurring adjustments, necessary for the fair statement of the Company’s financial position at March 31, 2014 and results of its operations for the three months ended March 31, 2014 and 2013, and its cash flows for the three months ended March 31, 2014 and 2013. The results for three months ended March 31, 2014 are not necessarily indicative of the results to be expected for the year ending December 31, 2014 or for any other future annual or interim period.
Use of estimates
The preparation of consolidated condensed interim financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions about future events that affect the reported amounts of assets, liabilities, expenses, contingent assets and contingent liabilities as at the end or during the reporting period. Actual results could significantly differ from those estimates. Significant areas requiring management to make estimates include the derivative liability and the valuation of equity instruments issued for services. There have been no changes to the methodology used in determining these estimates from the year ended December 31, 2013.
Loss per share
Loss per share is calculated based on the weighted average number of common shares outstanding. For the three month periods ended March 31, 2014 and March 31, 2013 respectively, diluted loss per share does not differ from basic loss per share since the effect of the Company’s warrants and stock options are anti-dilutive. At March 31, 2014, potential common shares of 22,392,696 (March 31, 2013 – 24,985,009) relating to warrants and 3,240,000 (March 31, 2013 - 1,020,000) relating to stock options were excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
Recent accounting pronouncements
The Company reviews new accounting standards as issued.
The accounting pronouncements issued subsequent to the date of these financial statements that were considered significant by management were evaluated for the potential effect on these financial statements. Management does not believe any of the subsequent pronouncements will have a material effect on these financial statements as presented and does not anticipate the need for any future restatement of these financial statements because of the retro-active application of any accounting pronouncements issued subsequent to March 31, 2014 through the date these financial statements were issued.
|
3
|
Related party transactions
|
During the three months ended March 31, 2014
Pursuant to consulting agreements with the Company’s officers the Company pays a total of $32,000 per month in cash compensation to its officers. Pursuant to these agreements the Company recognized a total of $96,000 in compensation expense for the three months ended March 31, 2014.
Included in accounts payable at March 31, 2014 is an aggregate amount owing of $35,798 (December 31, 2013 - $74,754) to the Company’s officers and directors for fees and expenses. The Company pays related party payables incurred for fees and expenses under normal commercial terms.
The Company paid $24,000 in directors’ fees during the three months ended March 31, 2014.
During the three months ended March 31, 2013
Pursuant to consulting agreements with the Company’s officers and directors the Company pays a total of $36,784 per month to its officers and directors. Pursuant to these agreements the Company recognized a total of $110,352 in compensation expense for the three months ended March 31, 2013.
On January 25, 2013, in connection with the Reverse Acquisition (note 1), Valent was issued 1,150,000 shares of common stock of the Company in exchange for Valent agreeing to the reduction of certain future royalties payable to Valent under its Assignment Agreement with the Company. As a result of the share issuance the Company has recognized an expense of $598,000 for the three months ended March 31, 2013.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
The Company has issued stock purchase warrants. Based on the terms of certain of these warrants the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value each reporting period with the changes in fair value recorded in the consolidated condensed statement of loss and comprehensive loss.
CDN $0.50 Unit Warrants
During the three months ended March 31, 2014, 20,000 warrants were exercised for no additional consideration for 20,000 shares of common stock. As a result, $17,600 of the derivative liability has been reclassified to equity. The warrants that have been exercised were revalued at their exercise date and then the reclassification to equity was recorded.
The remaining 2,169,000 warrants expired on January 25, 2014. As of March 31, 2014 there are no CDN $0.50 unit warrants outstanding.
Investor Warrants
During the three months ended March 31, 2014, 277,313 warrants were exercised for 277,313 shares of common stock. The Company received proceeds of $221,850 from the exercise. As a result, $126,064 of the derivative liability has been reclassified to equity. The warrants that have been exercised were revalued at their exercise date and then the reclassification to equity was recorded.
The remaining 12,847,689 Investor Warrants issued with the units have been re-valued at March 31, 2014 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility - 78%, risk free rate - 1.44% and a term of approximately four years.
Dividend Warrants
The Dividend Warrants have been measured at fair value at March 31, 2014 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility - 78%, risk free rate - 1.44% and a term of approximately four years.
Warrants issued for services
The Company has issued 300,000 warrants for services. The warrants were issued on September 12, 2013 and are exercisable on a cashless basis at an exercise price of $1.76 for five years. The warrants have been measured at March 31, 2014 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility -81%, risk free rate - 1.62% and a term of approximately 4.5 years.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
The Company’s derivative liability is summarized as follows:
| |
|
March 31,
2014
$
|
|
|
December 31,
2013
$
|
|
| |
|
|
|
|
|
|
|
Opening balance
|
|
|
4,402,306 |
|
|
|
121,000 |
|
| |
|
|
|
|
|
|
|
|
|
Issuance of units
|
|
|
- |
|
|
|
3,681,372 |
|
|
Dividend Warrant liability acquired on reverse acquisition
|
|
|
- |
|
|
|
2,041,680 |
|
|
Warrants issued for services
|
|
|
- |
|
|
|
124,020 |
|
|
Change in fair value of unexercised warrants
|
|
|
1,599,349 |
|
|
|
(1,324,051 |
) |
|
Reclassification to equity upon exercise of warrants
|
|
|
(143,664 |
) |
|
|
(241,715 |
) |
| |
|
|
|
|
|
|
|
|
|
Closing balance
|
|
|
5,857,991 |
|
|
|
4,402,306 |
|
|
5
|
Stockholders’ deficiency
|
Preferred stock
Authorized
5,000,000 preferred shares, $0.001 par value
Issued and outstanding at March 31, 2014 - 1 (December 31, 2013 - 1)
Common stock
Authorized
200,000,000 common shares, $0.001 par value
Issued and outstanding at March 31, 2014 – 32,082,132 (December 31, 2013 – 31,534,819).
The issued and outstanding common shares at March 31, 2014 include 7,169,583 shares of common stock on an as-exchanged basis with respect to the shares of Exchangeco that can be exchanged for shares of common stock of the Company.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
| |
|
Shares of common stock
outstanding
|
|
|
Common stock
|
|
|
Additional paid-in capital
|
|
|
Warrants
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance – December 31, 2013
|
|
|
31,534,819 |
|
|
|
31,535 |
|
|
|
8,791,715 |
|
|
|
6,202,100 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of Investor Warrants
|
|
|
277,313 |
|
|
|
277 |
|
|
|
221,573 |
|
|
|
- |
|
|
Exercise of CDN $0.50 unit warrants
|
|
|
20,000 |
|
|
|
20 |
|
|
|
17,580 |
|
|
|
- |
|
|
Shares issued for services (a)
|
|
|
250,000 |
|
|
|
250 |
|
|
|
314,750 |
|
|
|
- |
|
|
Reclassification of derivative liability to equity on exercise of warrants
|
|
|
- |
|
|
|
- |
|
|
|
126,064 |
|
|
|
- |
|
|
Expiry of broker warrants
|
|
|
- |
|
|
|
- |
|
|
|
556 |
|
|
|
(556 |
) |
|
Stock-based compensation
|
|
|
- |
|
|
|
- |
|
|
|
275,772 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance – March 31, 2014
|
|
|
32,082,132 |
|
|
|
32,082 |
|
|
|
9,748,010 |
|
|
|
6,201,544 |
|
|
a)
|
Shares issued for services
|
During the quarter ended March 31, 2014 the Company issued 250,000 shares of common stock for services rendered to the Company. The shares issued for services have been valued using the closing price of the Company’s common stock on the day the shares for services were issued. A total of $315,000 in general and administrative expense has been recognized for these shares for the three months ended March 31, 2014.
The total share-based payment expense of $620,074 (2013 - $272,902), including stock option expense of $305,074 (2013 - $204,345), has been recognized for the three months ended March 31, 2014. This total expense has been recognized as to $171,947 and $448,127 for research and development, and general and administrative respectively for the three months ended March 31, 2014. The prior period expense of $272,902 has been recognized as to $152,480 and $120,422 for research and development, and general and administrative respectively for the three months ended March 31, 2013.
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
Stock Options
The following table sets forth the options outstanding:
| |
|
Number of
stock
options
outstanding
|
|
|
Weighted
average
exercise
price
$
|
|
| |
|
|
|
|
|
|
|
Balance – March 31, 2014 and December 31, 2013
|
|
|
3,240,000 |
|
|
|
0.96 |
|
| |
|
|
|
|
|
|
|
|
The following table summarizes stock options currently outstanding and exercisable at March 31, 2014:
|
Exercise price
$
|
|
|
Number
outstanding at
March 31,
2014
|
|
|
Weighted
average
remaining
contractual
life
(years)
|
|
|
Weighted
average
exercise
price
$
|
|
|
Number
exercisable
at
March 31,
2014
|
|
|
Exercise
price
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
0.45 |
|
|
|
900,000 |
|
|
|
7.83 |
|
|
|
0.45 |
|
|
|
763,833 |
|
|
|
0.45 |
|
| |
1.05 |
|
|
|
2,040,000 |
|
|
|
9.37 |
|
|
|
1.05 |
|
|
|
970,963 |
|
|
|
1.05 |
|
| |
1.54 |
|
|
|
180,000 |
|
|
|
9.00 |
|
|
|
1.54 |
|
|
|
180,000 |
|
|
|
1.54 |
|
| |
2.30 |
|
|
|
120,000 |
|
|
|
9.17 |
|
|
|
2.30 |
|
|
|
100,000 |
|
|
|
2.30 |
|
| |
|
|
|
|
3,240,000 |
|
|
|
|
|
|
|
0.96 |
|
|
|
2,014,796 |
|
|
|
0.93 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Included in the number of stock options outstanding are 900,000 stock options granted at an exercise price of CDN $0.50. The exercise prices shown in the above table have been converted to $0.45 USD using the period ending closing exchange rate. Certain stock options have been granted to non-employees and will be revalued at each reporting date until they have fully vested. The stock options have been re-valued using a Black-Scholes pricing model using the following assumptions:
| |
|
March 31,
2014
|
|
| |
|
|
|
|
Dividend rate
|
|
|
0 |
% |
|
Volatility
|
|
72.8% to 75.5
|
% |
|
Risk-free rate
|
|
|
1.25 |
% |
|
Term - years
|
|
0.83 to 2.38
|
|
The Company has recognized the following amounts as stock-based compensation expense for the periods noted:
| |
|
Three months ended
March 31,
|
|
| |
|
|
|
|
|
|
| |
|
|
2014
$
|
|
|
|
2013
$
|
|
| |
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
171,947 |
|
|
|
152,480 |
|
|
General and administrative
|
|
|
133,127 |
|
|
|
51,865 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
305,074 |
|
|
|
204,345 |
|
DelMar Pharmaceuticals, Inc.
(a development stage company)
Notes to Consolidated Condensed Interim Financial Statements
(Unaudited)
(expressed in US dollars unless otherwise noted)
During the quarter ended March 31, 2013 the Company’s functional currency changed from $CDN to $USD. As a result, certain stock options previously granted by the Company are now recognized as a long-term liability. Of the total stock option expense of $305,074 (2013 - $204,345), $275,772 (2013 - $20,846) has been recognized as additional paid in capital and $29,302 (2013 - $183,499) has been recognized as a stock option liability.
The aggregate intrinsic value of stock options outstanding at March 31, 2014 was $1,008,330 (2013 - $1,181,007) and the aggregate intrinsic value of stock options exercisable at March 31, 2014 was $734,111 (2013 - $764,760). As of March 31, 2014 there was $273,996 in unrecognized compensation expense that will be recognized over the next 2.4 years. No stock options granted under the Plan have been exercised to March 31, 2014. Upon the exercise of stock options new shares will be issued.
A summary of status of the Company’s unvested stock options under all plans is presented below:
| |
|
Number of
Options
|
|
|
Weighted
average
exercise
price
$
|
|
|
Weighted
average
grant date
fair value
$
|
|
| |
|
|
|
|
|
|
|
|
|
|
Unvested at December 31, 2013
|
|
|
1,679,371 |
|
|
|
1.08 |
|
|
|
0.59 |
|
|
Vested
|
|
|
(454,167 |
) |
|
|
1.09 |
|
|
|
0.60 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unvested at March 31, 2014
|
|
|
1,225,204 |
|
|
|
1.07 |
|
|
|
0.59 |
|
Certain of the Company’s warrants have been recognized as a derivative liability (note 5). The following table summarizes all of the Company’s outstanding warrants as of March 31, 2014:
|
Description
|
|
Number
|
|
| |
|
|
|
|
Balance – December 31, 2013
|
|
|
24,864,009 |
|
|
CDN $0.50 unit warrants (i)
|
|
|
(2,189,000 |
) |
|
Broker warrants(ii)
|
|
|
(5,000 |
) |
|
Investor warrants (iii)
|
|
|
(277,313 |
) |
| |
|
|
|
|
|
Balance - March 31, 2014
|
|
|
22,392,696 |
|
|
i)
|
Of the balance of 2,189,000 outstanding at December 31, 2013, 20,000 were exercised for no additional consideration and 2,169,000 expired on January 25, 2014.
|
|
ii)
|
Broker warrants with an exercise price of CDN $0.50 expired on March 1, 2014. The fair value of the warrants of $556 has been reclassified from warrants to additional paid in capital at March 31, 2014.
|
|
iii)
|
During the three months ended March 31, 2014, 277,313 warrants were exercised for 277,313 shares of common stock. The Company received proceeds of $221,850 from the exercise.
|
The Company has financial instruments that are measured at fair value. To determine the fair value, we use the fair value hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use to value an asset or liability and are developed based on market data obtained from independent sources. Unobservable
inputs are inputs based on assumptions about the factors market participants would use to value an asset or liability. The three levels of inputs that may be used to measure fair value are as follows:
| ● |
|
Level one - inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities; |
| |
|
|
| ● |
|
Level two - inputs are inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly such as interest rates, foreign exchange rates, and yield curves that are observable at commonly quoted intervals; and |
| |
|
|
| ● |
|
Level three - unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use. |
Assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. Changes in the observability of valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy.
The Company’s financial instruments consist of cash and cash equivalents, other receivables, accounts payable, related party payables and derivative liability. The carrying values of cash and cash equivalents, other receivables, accounts payable and related party payables approximate their fair values due to the immediate or short-term maturity of these financial instruments.
As quoted prices for the derivative liability are not readily available, the Company has used a simulated probability valuation model, as described in note 2 to estimate fair value. The derivative liability utilizes Level 3 inputs as defined above.
The Company has the following liabilities under the fair value hierarchy:
| |
|
|
|
|
|
|
|
March 31, 2014
|
|
| |
|
|
|
|
|
|
|
|
|
|
Liability
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
| |
|
|
|
|
|
|
|
|
|
|
Derivative liability
|
|
|
- |
|
|
|
- |
|
|
|
5,857,991 |
|
| |
|
|
|
|
|
|
|
|
December 31, 2013
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Liability
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
| |
|
|
|
|
|
|
|
|
|
|
Derivative liability
|
|
|
- |
|
|
|
- |
|
|
|
4,402,306 |
|
Warrant exercises
Subsequent to March 31, 2014, the Company issued 8,000 shares of common stock pursuant to the exercise of warrants. The warrants were exercised at CDN $0.50 per warrant for proceeds of CDN $4,000.
Share issuance
Subsequent to March 31, 2014, the Company issued 250,000 shares of common stock for services to an unrelated company.
Exhibit C
DELMAR PHARMACEUTICALS, INC.
PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
(Expressed in United States Dollars, except where specified otherwise)
MARCH 31, 2014
DELMAR PHARMACEUTICALS, INC
PRO FORMA CONSOLIDATED BALANCE SHEET
AS AT MARCH 31, 2014
(Unaudited)
(Expressed in United States Dollars, except where specified otherwise)
|
Assets
|
|
|
DelMar Pharmaceuticals Inc.
|
|
Notes |
|
|
Pro forma adjustments
|
|
|
Pro forma consolidated
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Current Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
|
3,474,150 |
|
|
|
4 |
(a)(i) |
|
|
7,933,448 |
|
|
|
11,407,598 |
|
|
Taxes and other receivables
|
|
|
10,419 |
|
|
|
|
|
|
|
- |
|
|
|
10,419 |
|
|
Prepaid expenses
|
|
|
269,584 |
|
|
|
|
|
|
|
- |
|
|
|
269,584 |
|
|
Total assets
|
|
|
3,754,153 |
|
|
|
|
|
|
|
7,933,448 |
|
|
|
11,687,601 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities
|
|
|
404,413 |
|
|
|
4 |
(a)(v) |
|
|
36,000 |
|
|
|
440,413 |
|
|
Related party payables
|
|
|
35,798 |
|
|
|
|
|
|
|
- |
|
|
|
35,798 |
|
| |
|
|
440,211 |
|
|
|
|
|
|
|
36,000 |
|
|
|
476,211 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loan payable to Valent
|
|
|
274,387 |
|
|
|
|
|
|
|
- |
|
|
|
274,387 |
|
|
Stock option liability
|
|
|
241,863 |
|
|
|
|
|
|
|
- |
|
|
|
241,863 |
|
|
Derivative liability
|
|
|
5,857,991 |
|
|
4
|
(a)(iii) |
|
|
(4,669,731 |
) |
|
|
1,618,260 |
|
| |
|
|
|
|
|
4
|
(a)(iv) |
|
|
430,000 |
|
|
|
|
|
| |
|
|
6,814,452 |
|
|
|
|
|
|
|
(4,203,731 |
) |
|
|
2,610,721 |
|
|
Stockholders’ Deficiency
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock
|
|
|
32,082 |
|
|
|
4 |
(a)(i) |
|
|
12,848 |
|
|
|
44,930 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
9,748,010 |
|
|
|
4 |
(a)(i) |
|
|
8,338,150 |
|
|
|
22,338,341 |
|
| |
|
|
|
|
|
4
|
(a)(ii) |
|
|
(417,550 |
) |
|
|
|
|
| |
|
|
|
|
|
4
|
(a)(iii) |
|
|
4,669,731 |
|
|
|
|
|
|
Warrants
|
|
|
6,201,544 |
|
|
|
|
|
|
|
|
|
|
|
6,201,544 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deficit accumultaed during the development stage
|
|
|
(19,063,113 |
) |
|
4
|
(a)(iv) |
|
|
(430,000 |
) |
|
|
(19,529,113 |
) |
| |
|
|
|
|
|
|
4 |
(a)(v) |
|
|
(36,000 |
) |
|
|
|
|
|
Accumulated other comprehensive income
|
|
|
21,178 |
|
|
|
|
|
|
|
|
|
|
|
21,178 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
(3,060,299 |
) |
|
|
|
|
|
|
12,137,179 |
|
|
|
9,076,880 |
|
| |
|
|
3,754,153 |
|
|
|
|
|
|
|
7,933,448 |
|
|
|
11,687,601 |
|
The accompanying notes are an integral part of these Pro Forma Consolidated Financial Statements.
DELMAR PHARMACEUTICALS, INC.
PRO FORMA CONSOLIDATED STATEMENT OF LOSS AND COMPREHENSIVE LOSS
FOR THE THREE MONTHS ENDED MARCH 31, 2014
(Unaudited)
(Expressed in United States Dollars, except where specified otherwise)
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
DelMar Pharmaceuticals Inc.
|
|
|
Notes
|
|
|
Pro forma adjustments
|
|
|
Pro forma consolidated
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenses
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Research and development
|
|
|
618,869 |
|
|
|
|
|
|
|
|
|
|
|
618,869 |
|
|
General and administrative
|
|
|
966,923 |
|
|
|
4 |
(a)(v) |
|
|
36,000 |
|
|
|
1,002,923 |
|
| |
|
|
1,585,792 |
|
|
|
|
|
|
|
36,000 |
|
|
|
1,621,792 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other loss (income)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of derivative liability
|
|
|
1,599,349 |
|
|
|
4
|
(a)(iv) |
|
|
430,000 |
|
|
|
2,029,349 |
|
|
Foreign exchange (loss) gain
|
|
|
11,947 |
|
|
|
|
|
|
|
|
|
|
|
11,947 |
|
|
Interest expense
|
|
|
2,015 |
|
|
|
|
|
|
|
|
|
|
|
2,015 |
|
|
Interest income
|
|
|
(496 |
) |
|
|
|
|
|
|
|
|
|
|
(496 |
) |
| |
|
|
1,612,815 |
|
|
|
|
|
|
|
430,000 |
|
|
|
2,042,815 |
|
|
Loss from continuing operations before nonrecurring charges or credits directly attributable to the transaction
|
|
|
3,198,607 |
|
|
|
|
|
|
|
466,000 |
|
|
|
3,664,607 |
|
|
Pro forma weighted average number of shares outstanding - basic and diluted
|
|
|
31,659,791 |
|
|
|
|
|
|
|
|
|
|
|
44,507,480 |
|
| Pro forma adjusted loss per share - basic and diluted |
|
$ |
(0.10 |
) |
|
|
|
|
|
|
|
|
|
$ |
(0.08 |
) |
The accompanying notes are an integral part of these pro-forma consolidated financial statements.
DELMAR PHARMACEUTICALS, INC.
PRO FORMA CONSOLIDATED STATEMENT OF LOSS AND COMPREHENSIVE LOSS
FOR THE YEAR ENDED DECEMBER 31, 2013
(Unaudited)
(Expressed in United States Dollars, except where specified otherwise)
| |
|
DelMar Pharmaceuticals Inc.
|
|
|
Notes
|
|
|
Pro forma adjustments
|
|
|
Pro forma consolidated
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenses
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
Research and development
|
|
|
2,342,654 |
|
|
|
|
|
|
|
|
|
|
|
2,342,654 |
|
|
General and administrative
|
|
|
3,952,307 |
|
|
|
4 |
(a)(v) |
|
|
36,000 |
|
|
|
3,988,307 |
|
| |
|
|
6,294,961 |
|
|
|
|
|
|
|
36,000 |
|
|
|
6,330,961 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other loss (income)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of derivative liability
|
|
|
(1,324,051 |
) |
|
|
4
|
(a)(iv) |
|
|
315,000 |
|
|
|
(1,009,051 |
) |
|
Issuance of shares to Valent for future royalty reduction
|
|
|
598,000 |
|
|
|
|
|
|
|
|
|
|
|
598,000 |
|
|
Derivative issue costs
|
|
|
2,713,220 |
|
|
|
|
|
|
|
|
|
|
|
2,713,220 |
|
|
Foreign exchange (loss) gain
|
|
|
3,030 |
|
|
|
|
|
|
|
|
|
|
|
3,030 |
|
|
Interest expense
|
|
|
8,020 |
|
|
|
|
|
|
|
|
|
|
|
8,020 |
|
|
Interest income
|
|
|
(2,491 |
) |
|
|
|
|
|
|
|
|
|
|
(2,491 |
) |
| |
|
|
1,995,728 |
|
|
|
|
|
|
|
315,000 |
|
|
|
2,310,728 |
|
|
Loss from continuing operations before nonrecurring charges or credits directly attributable to the transaction
|
|
|
8,290,689 |
|
|
|
|
|
|
|
351,000 |
|
|
|
8,641,689 |
|
|
Pro forma weighted average number of shares outstanding - basic and diluted
|
|
|
29,667,324 |
|
|
|
|
|
|
|
|
|
|
|
42,515,013 |
|
|
Pro forma adjusted loss per share - basic and diluted
|
|
$ |
(0.28 |
) |
|
|
|
|
|
|
|
|
|
$ |
(0.20 |
) |
The accompanying notes are an integral part of these pro-forma consolidated financial statements.
DELMAR PHARMACEUTICALS, INC.
NOTES TO PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
(Expressed in United States Dollars, except where specified otherwise)
March 31, 2014
1. BASIS OF PRESENTATION
The accompanying unaudited pro forma consolidated balance sheet as at March 31, 2014 and the unaudited pro forma consolidated statements of loss and comprehensive loss for the three month period ended March 31, 2014 (the “Pro Forma Consolidated Financial Statements”) of DelMar Pharmaceuticals, Inc. ("DelMar") have been prepared by management on the basis of United States Generally Accepted Accounting Principles (“US GAAP”) and in accordance with the rules and regulations of the United States Securities and Exchange Commission (“SEC”) from information derived from the financial statements of DelMar. The unaudited Pro Forma Consolidated Financial Statements have been prepared for inclusion in the Schedule TO in conjunction with the tender offer to reduce the exercise price of certain DelMar warrants from $0.80 per warrant to $0.65 per warrant (the “Tender Offer”).
The unaudited pro forma balance sheet as at March 31, 2014 has been prepared as if 100% of the Investor Warrants outstanding on March 31, 2014 are exercised on March 31, 2014. The unaudited pro forma statement of loss and comprehensive loss for the three months ended March 31, 2014 has been prepared as if all of the Investor Warrants outstanding on March 31, 2014 had been exercised on January 1, 2014. The unaudited pro forma statement of loss and comprehensive loss for the year ended December 31, 2013 has been prepared as if all of the Investor Warrants outstanding on March 31, 2014 have been exercised on January 1, 2013.
The unaudited Pro Forma Consolidated Financial Statements have been derived from:
a) the unaudited condensed interim financial statements of DelMar for three month period ended March 31, 2014.
The unaudited pro forma adjustments are based on currently available information and certain assumptions that management believes are reasonable. The unaudited Pro Forma Consolidated Financial Statements should be read in conjunction with the selected historical financial information and related financial statements and accompanying footnotes of DelMar. The unaudited Pro Forma Consolidated Financial Statements are for informational purposes only and do not purport to reflect the financial position or results of operations that would have occurred if the Tender Offer had been consummated on the dates indicated above, nor do they purport to represent or be indicative of the financial position or results of operations of DelMar for any future dates or periods. Unless otherwise stated, all amounts presented in these unaudited Pro Forma Consolidated Financial Statements are in U.S. dollars.
2. SIGNIFICANT ACCOUNTING POLICIES
The accounting policies used in the preparation of these unaudited Pro Forma Consolidated Financial Statements are those set out in DelMar’s audited financial statements for the year ended December 31, 2013 and DelMar’s unaudited condensed interim financial statements for the three months ended March 31, 2014.
DELMAR PHARMACEUTICALS, INC.
NOTES TO PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
(Expressed in United States Dollars, except where specified otherwise)
March 31, 2014
3. DESCRIPTION OF THE TRANSACTION
DelMar is offering to amend warrants to purchase an aggregate of 9,195,478 shares of common stock (the “Offer to Amend and Exercise”), including outstanding warrants to purchase 9,195,478 shares of DelMar’s common stock issued to investors participating in Delmar’s private placement financings closed on January 25, 2013, January 31, 2013, February 8, 2013, February 21, 2013, February 28, 2013, March 1, 2013, and March 6, 2013 (the “Investor Warrants”). DelMar initially issued a total of 13,125,002 Investor Warrants. Of these 13,125,002 Investor Warrants, 277,313 were exercised during the quarter ended March 31, 2014 and 3,652,211 were exercised on June 6, 2014. As a result, 9,195,478 are currently outstanding and are included in the Tender Offer. The 3,652,211 Investor Warrants exercised on June 6, 2014 are not part of the Tender Offer but have been included in the Pro Forma Consolidated Financial Statements as DelMar is presenting its unaudited Pro Forma Consolidated Financial Statements as of March 31, 2014. The 3,652,211 Investor Warrants exercised on June 6, 2014 have been treated as if they were exercised March 31, 2014 for presentation in the unaudited pro forma balance sheet and as if they were exercised on January 1, 2014 for presentation in the unaudited pro forma statement of loss and comprehensive loss for the three months ended March 31, 2014. The unaudited pro forma statement of loss and comprehensive loss for the year ended December 31, 2013 has been prepared as if all of the Investor Warrants outstanding on March 31, 2014 had been exercised on January 1, 2013.
There is no minimum participation requirement with respect to the Offer to Amend and Exercise. Pursuant to the Offer to Amend and Exercise, the Investor Warrants will be amended (the “Amended Warrants” ) to: (i) reduce the exercise price of the Investor Warrants from $0.80 per share to $0.65 per share of common stock in cash, (ii) shorten the exercise period of the Investor Warrants so that they expire concurrently with the expiration of the Offer to Amend and Exercise at 5:00 p.m. (Pacific Time) on July 28, 2014, as may be extended by DelMar in its sole discretion (“ Expiration Date ”), (iii) delete the price-based anti-dilution provisions contained in the Investor Warrants, (iv) restrict the ability of the holder of shares issuable upon exercise of the Amended Warrants to sell, make any short sale of, loan, grant any option for the purchase of, or otherwise dispose of any of such shares without the prior written consent of DelMar for a period of time twenty (20) days after the Expiration Date (the “Lock-Up Period”); and (v) provide that a holder, acting alone or with others, will agree not to effect any purchases or sales of any securities of DelMar in any “short sales” as defined in Rule 200 promulgated under Regulation SHO under the Exchange Act, or any type of direct and indirect stock pledges, forward sale contracts, options, puts, calls, short sales, swaps, “put equivalent positions” (as defined in Rule 16a-1(h) under the Exchange Act) or similar arrangements, or sales or other transactions through non-U.S. broker dealers or foreign regulated brokers through the expiration of the Lock-Up Period. Other than set forth above the terms of the Investor Warrants will remain unmodified and in full force and effect.
Holders may elect to amend some or all of their Investor Warrants. If a holder chooses not to participate in the Offer to Amend and Exercise, the respective Investor Warrants will remain in full force and effect, as originally issued. The purpose of the Offer to Amend and Exercise is to encourage the amendment and exercise of the Investor Warrants to help DelMar reduce its outstanding warrant liability and to raise funds to support DelMar’s operations by providing the holders of the Investor Warrants with the opportunity to obtain and exercise an Amended Warrant by significantly reducing the exercise price of the Investor Warrants.
i) Warrants
A total of 13,125,002 Investor Warrants were issued by DelMar as part of its private placement financings closed on January 25, 2013, January 31, 2013, February 8, 2013, February 21, 2013, February 28, 2013, March 1, 2013, and March 6, 2013. As of March 31, 2014, 277,313 Investor Warrants have been exercised at a price of $0.80 per Investor Warrant for 277,313 shares of common stock. The Company received proceeds of $221,850 from the exercises. On June 6, 2014, 3,652,211 Investor Warrants were exercised at a price of $0.65 per Investor Warrant for 3,652,211 shares of common stock. The Company received gross proceeds of $2,373,937 from the exercises. The unaudited pro forma balance sheet gives effect to the exercise of 12,847,689 Investor Warrants outstanding as at March 31, 2014 at $0.65 per Investor Warrant for gross proceeds of $8,350,998.
DELMAR PHARMACEUTICALS, INC.
NOTES TO PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
(Expressed in United States Dollars, except where specified otherwise)
March 31, 2014
DESCRIPTION OF THE TRANSACTION (continued)
ii) Derivative liability
Based on the terms of the Investor Warrants, DelMar determined that the Investor Warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value each reporting period with the changes in fair value recorded in the consolidated statement of loss and comprehensive loss. If all of the Investor Warrants outstanding at March 31, 2014 are exercised, the entire derivative liability of $4,669,731 associated with the Investor Warrants would be reclassified to additional paid-in capital at March 31, 2014.
The derivative liability associated with certain of DelMar’s other warrants would still continue to be recognized. DelMar currently incorporates a liquidity discount factor when calculating the fair value of its derivative liability. As a result of assuming 100% of the Investor Warrants have been exercised, the liquidity discount factor would need to be adjusted when being applied to the remaining derivative liability. As a result, an increase in the remaining derivative liability of $430,000 and $315,000 at March 31, 2014 and December 31, 2013 respectively, would be recognized.
iii) Warrant agent commission
National Securities Corporation will receive a fee equal to 5% of the cash exercise prices paid by holders of the Investor Warrants who participate in the Offer to Amend and Exercise. In addition, DelMar has agreed to reimburse National Securities Corporation for its reasonable out-of-pocket expenses. If such expenses and fees exceed $1,000, National Securities Corporation must obtain DelMar’s prior approval.
iv) Other closing costs
Other closing costs of $36,000 are expected to be incurred to complete the Tender Offer.
4. PRO FORMA ASSUMPTIONS AND ADJUSTMENTS
The unaudited Pro Forma Consolidated Financial Statements are presented as if all of the 12,847,689 Investor Warrants outstanding at March 31, 2014 have been exercised at $0.65 per Investor Warrant on March 31, 2014. The following adjustments are directly attributable to the transaction:
| i) |
|
To record the exercise of 12,847,689 Investor Warrants for 12,847,689 shares of common stock for gross proceeds of $8,350,998. |
| ii) |
|
To record the 5% Warrant Agent commission of $417,550. DelMar would receive net proceeds of $7,933,448. |
| iii) |
|
To reclassify the derivative liability associated with the Investor Warrants to additional paid-in capital. |
| iv) |
|
To record change in remaining derivative liability. |
| iv) |
|
To record estimated additional transaction costs related to the Tender Offer of $36,000. |
DELMAR PHARMACEUTICALS, INC.
NOTES TO PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
(Expressed in United States Dollars, except where specified otherwise)
March 31, 2014
5. PRO FORMA COMMON STOCK
Pro forma common stock as at March 31, 2014 has been determined as follows:
| |
|
March 31, 2014
|
|
| |
|
Common Shares
|
|
|
Amount ($)
|
|
|
Common stock of DelMar at March 31, 2014
|
|
|
32,082,132 |
|
|
|
32,082 |
|
|
Shares issued on Investor Warrant exercise
|
|
|
12,847,689 |
|
|
|
12,848 |
|
|
Pro forma common stock at March 31, 2014
|
|
|
44,929,821 |
|
|
|
44,930 |
|
Book value per share at March 31, 2014 was ($0.095). The pro forma book value per share at March 31, 2014 is $0.20.
6. PRO FORMA LOSS PER SHARE
Pro forma loss per share has been determined as follows:
| |
|
Three months ended March 31, 2014
|
|
|
Weighted average number of DelMar common shares
|
|
|
31,659,791 |
|
|
Shares issued on Investor Warrant exercises
|
|
|
12,847,689 |
|
|
Pro forma weighted average number of shares outstanding - basic and diluted
|
|
|
44,507,480 |
|
|
Pro forma adjusted net loss
|
|
$ |
3,664,607 |
|
| Pro forma adjusted loss per share - basic and diulted |
|
$ |
(0.08 |
) |
| |
|
Year ended December 31, 2013
|
|
|
Weighted average number of DelMar common shares
|
|
|
29,667,324 |
|
|
Shares issued on Investor Warrant exercises
|
|
|
12,847,689 |
|
|
Pro forma weighted average number of shares outstanding - basic and diluted
|
|
|
42,515,013 |
|
|
Pro forma adjusted net loss
|
|
$ |
8,641,689 |
|
| Pro forma adjusted loss per share - basic and diulted |
|
$ |
(0.20 |
) |
C-8