The information in this proxy statement/prospectus is not complete and may be changed. These securities described herein may not be sold until the registration statement filed with the U.S. Securities and Exchange Commission is declared effective. This proxy statement/prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PRELIMINARY PROXY STATEMENT/PROSPECTUS
SUBJECT TO COMPLETION, DATED AUGUST 8, 2024

KINTARA THERAPEUTICS, INC.
9920 Pacific Heights Blvd, Suite 150
San Diego, CA 92130
To the Stockholders of Kintara Therapeutics, Inc. and TuHURA Biosciences, Inc.,
On behalf of the Kintara Therapeutics, Inc. (“Kintara”) board of directors, we are pleased to enclose the proxy statement/prospectus for the proposed acquisition of TuHURA Biosciences, Inc. by Kintara and the related facilitating transactions.
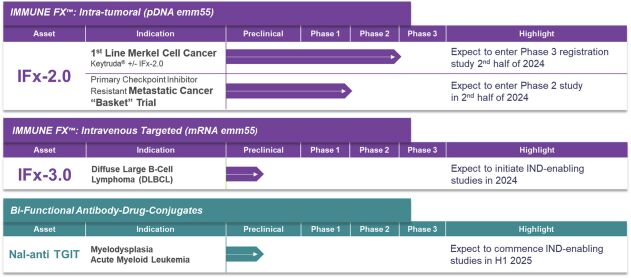
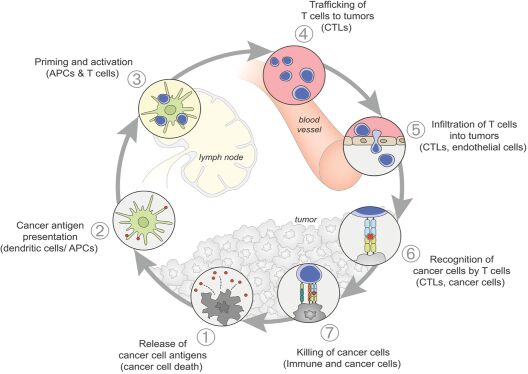
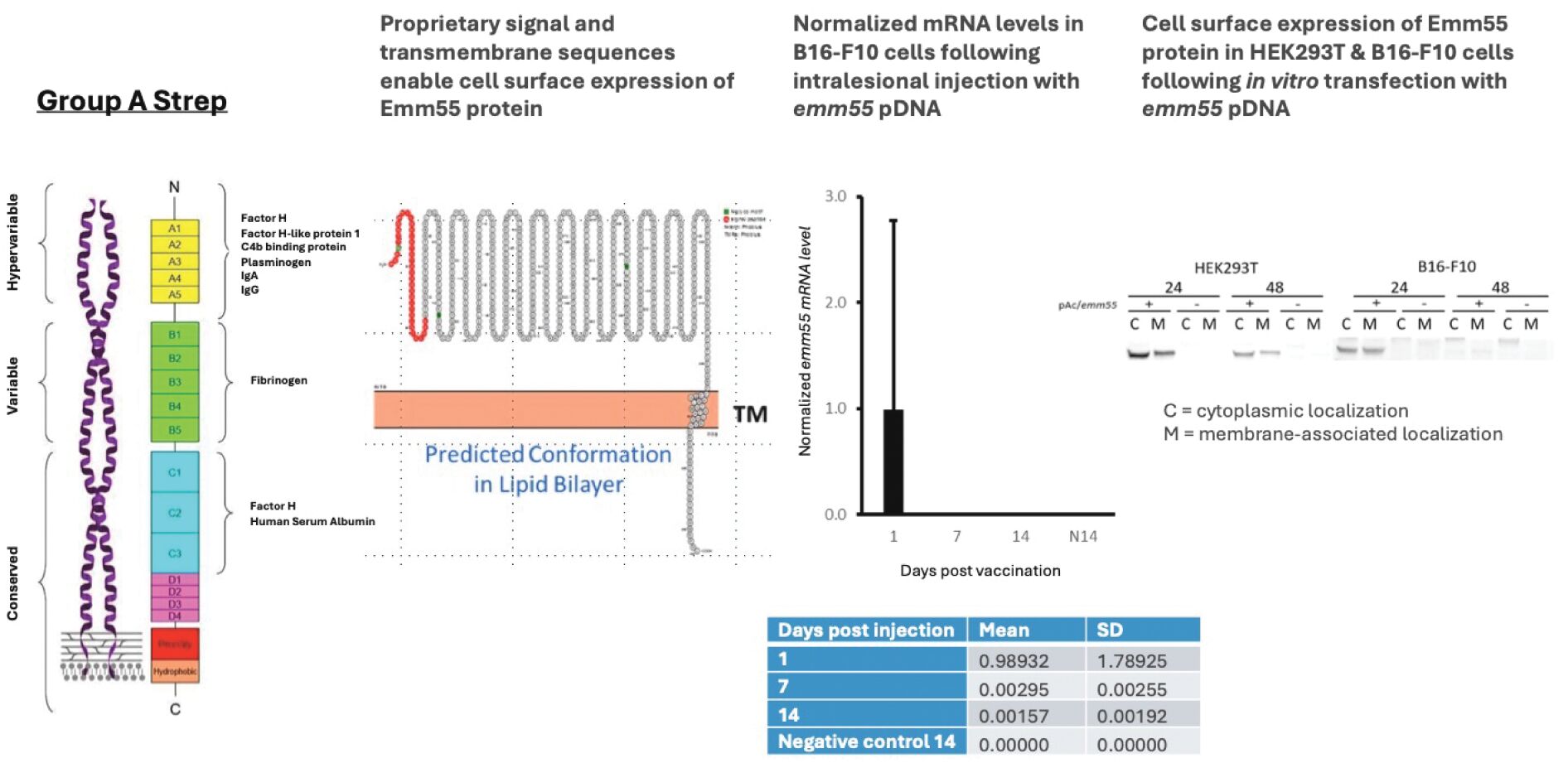
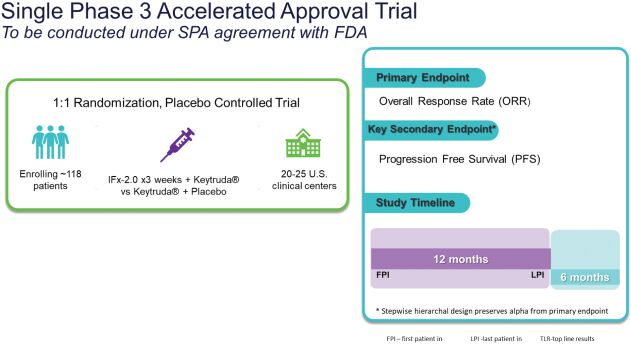
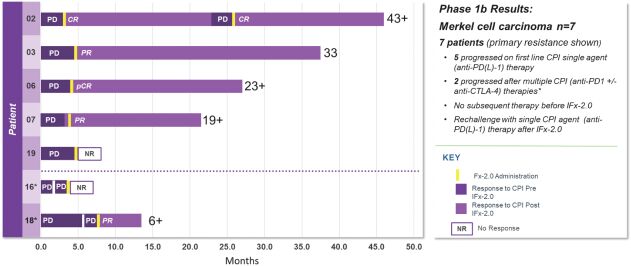
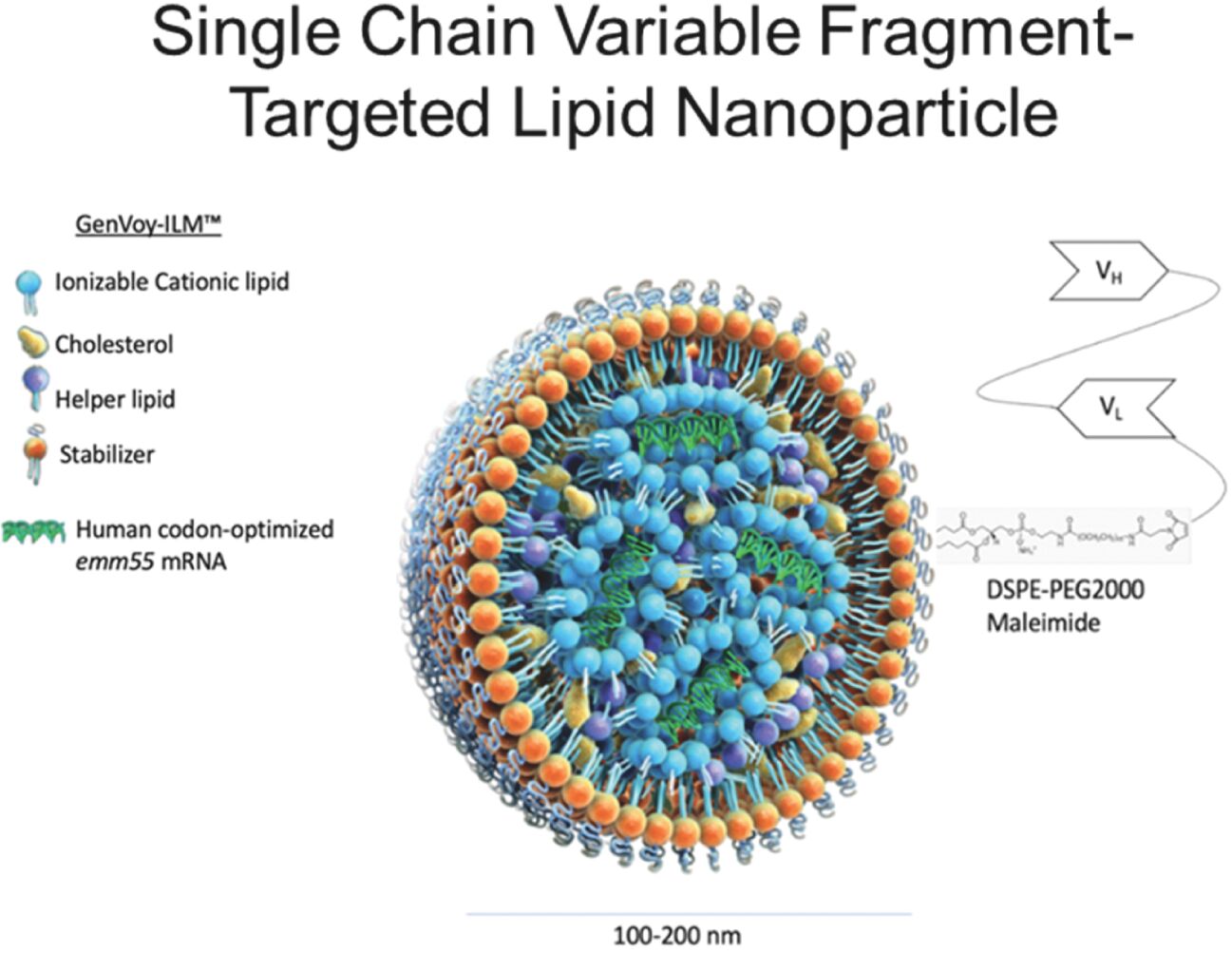
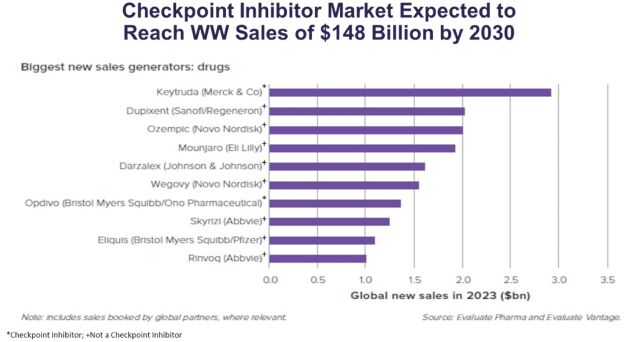
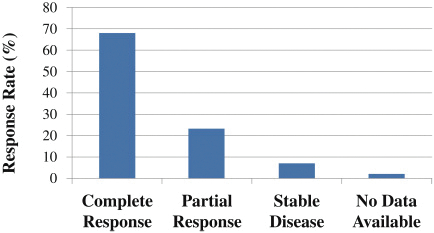
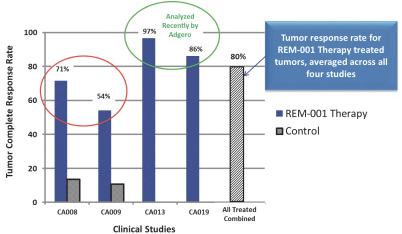
As previously announced, Kintara, Kayak Mergeco, Inc., a wholly-owned subsidiary of Kintara incorporated in the State of Delaware (“Merger Sub”), and TuHURA Biosciences, Inc., a Delaware corporation (“TuHURA”), entered into an Agreement and Plan of Merger, dated April 2, 2024 (the “Merger Agreement”) pursuant to which Merger Sub will merge with and into TuHURA, with TuHURA surviving the merger and becoming a wholly-owned direct subsidiary of Kintara (the “Merger”). The Merger will result in a publicly-traded company focused on advancing TuHURA’s personalized cancer vaccine(s) and bi-functional antibody drug conjugates, two technologies that seek to overcome the major obstacles that limit the effectiveness of current immunotherapies in treating cancer. In addition, the combined company will continue the REM-001 program, which is currently enrolled in an NIH-sponsored and funded open label 15-patient study in cutaneous metastatic breast cancer. Once 10 patients are enrolled and tracked in this study to determine whether a lower dose of REM-001 has an acceptable safety profile and elicits a treatment effect similar to that seen in prior REM-001 studies, the combined company expects to enroll the remaining patients and complete the trial and thereafter evaluate whether the REM-001 technology has potential future value that could be realized by the combined company.
Subject to the terms and conditions of the Merger Agreement, at the closing of the Merger, (a) each then-outstanding share of common stock, par value $0.001 per share, of TuHURA (the “TuHURA Common Stock”) (other than shares held in treasury and Dissenting Shares (as defined in the Merger Agreement)) will be converted into and become exchangeable for a number of shares of common stock, par value $0.001 per share, of Kintara (the “Kintara Common Stock”) calculated in accordance with the Merger Agreement, (b) each then-outstanding option to purchase TuHURA Common Stock will be assumed and converted by Kintara into an option to purchase shares of Kintara Common Stock, subject to certain adjustments as set forth in the Merger Agreement, and (c) each then-outstanding warrant to purchase shares of TuHURA Common Stock will be converted into and exchangeable for a warrant of like tenor entitling the holder to purchase shares of Kintara Common Stock, subject to certain adjustments as set forth in the Merger Agreement.
As a result of the Merger, existing equityholders of TuHURA will own approximately 97.15% of the combined company (or 94.55% of the combined company after giving effect to the issuance of the shares underlying the contingent value rights to be received by certain of Kintara’s equityholders) and the existing equityholders of Kintara would own approximately 2.85% of the combined company (or 5.45% of the combined company after giving effect to the issuance of the shares underlying the contingent value rights to be received by certain of Kintara’s equityholders). See the section entitled “The Merger Agreement” on page 161 of the attached proxy statement/prospectus for further information on the consideration being paid to the equityholders of TuHURA. Kintara’s Common Stock is currently listed on the Nasdaq Capital Market, under the symbol “KTRA.” After completion of the Merger, Kintara will be renamed “TuHURA Biosciences, Inc.” and it is expected that the common stock of the combined company will trade on the Nasdaq Capital Market under the symbol “HURA.”
The consummation of the Merger is conditioned on, among other things, the listing approval for the combined company’s securities on the Nasdaq Capital Market. Pursuant to the Merger Agreement, Kintara agreed, subject to Kintara stockholder approval, to effect a reverse stock split to ensure the Kintara Common Stock will be able to meet the $4.00 minimum bid price initial listing requirement.