
Exhibit 99.1

Copyright 2017, DelMar Pharmaceuticals All rights reserved Seeking New Horizons for Cancer Patients Corporate Update NASDAQ: DMPI October 4, 2017

Copyright 2017, DelMar Pharmaceuticals All rights reserved Forward - Looking Statements Any statements contained in this presentation that do not describe historical facts may constitute forward - looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995 and Canadian securities laws . Any forward - looking statements contained herein or made in the course of the presentation are based on current expectations, but are subject to a number of risks and uncertainties . The factors that could cause actual future results to differ materially from current expectations include, but are not limited to, risks and uncertainties relating to the Company's ability to develop, market and sell products based on its technology ; the expected benefits and efficacy of the Company's products and technology ; the availability of substantial additional funding for the Company to continue its operations and to conduct research and development, clinical studies and future product commercialization ; and, the Company's business, research, product development, regulatory approval, marketing and distribution plans and strategies . These and other factors are identified and described in more detail in our filings with the SEC and the British Columbia Securities Commission, including our current reports on Form 8 - K’s, Form 10 - Q’s and most recent Form 10 - K . We do not undertake to update these forward - looking statements made by us . 2

Copyright 2017, DelMar Pharmaceuticals All rights reserved Agenda for Today’s Call • Introductions • Recent Highlights • Overview of Financial Results • Update on VAL - 083 Clinical Research Programs 3

Copyright 2017, DelMar Pharmaceuticals All rights reserved Introductions - Management 4 Jeffrey A. Bacha, BSc MBA President & Chief Executive Officer Dennis Brown, PhD Chief Scientific Officer Scott Praill, CPA Chief Financial Officer

Copyright 2017, DelMar Pharmaceuticals All rights reserved Introductions – VAL - 083 A "first - in - class" DNA - targeting agent with a novel mechanism of action and the potential to overcome current therapy disadvantages • Clinical activity demonstrated in multiple NCI - sponsored clinical trials • Validated, unique mechanism of action differentiates VAL - 083 from other chemotherapies • Readily crosses the blood - brain - barrier • Biomarker - driven patient selection reduces clinical risk and enhances commercial positioning • Safety database of more than 1000 patients • Optimized dosing improves therapeutic window • Targeting GBM and ovarian cancer as initial opportunities
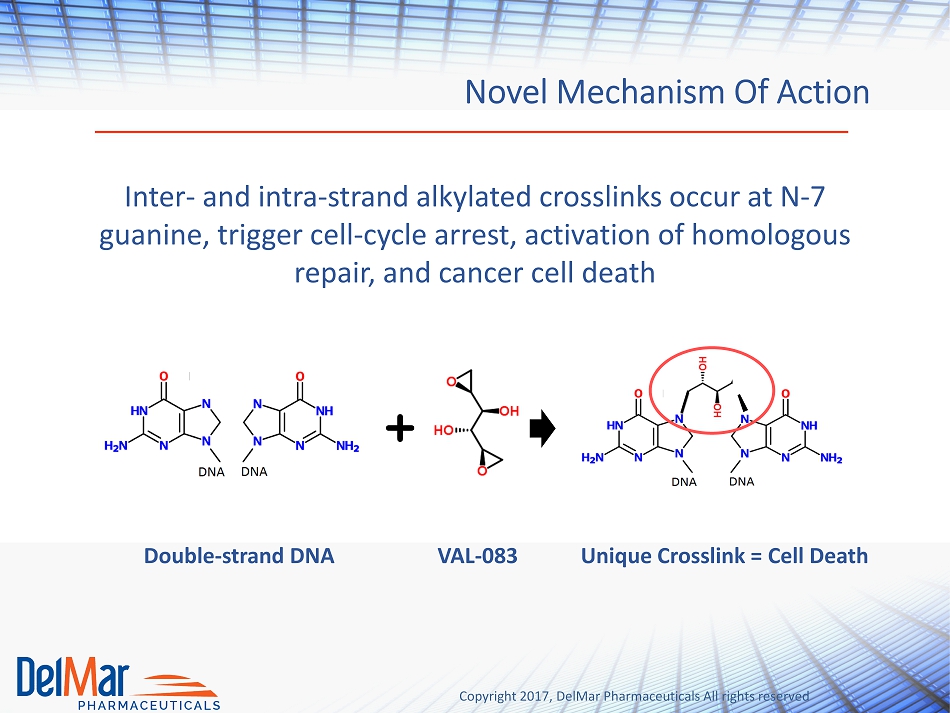
Copyright 2017, DelMar Pharmaceuticals All rights reserved Novel Mechanism Of Action Inter - and intra - strand alkylated crosslinks occur at N - 7 guanine, trigger cell - cycle arrest, activation of homologous repair, and cancer cell death Double - strand DNA VAL - 083 Unique Crosslink = Cell Death
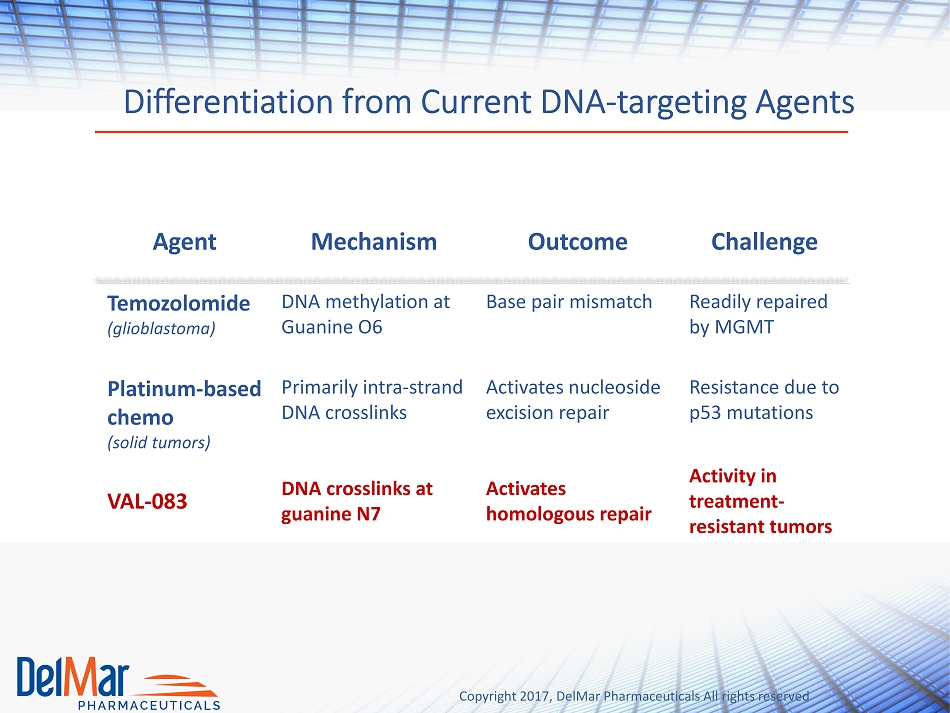
Copyright 2017, DelMar Pharmaceuticals All rights reserved Differentiation from Current DNA - targeting Agents Agent Mechanism Outcome Challenge Temozolomide (glioblastoma) DNA methylation at Guanine O6 Base pair mismatch Readily repaired by MGMT Platinum - based chemo (solid tumors) Primarily intra - strand DNA crosslinks Activates nucleoside excision repair Resistance due to p53 mutations VAL - 083 DNA crosslinks at guanine N7 Activates homologous repair Activity in treatment - resistant tumors
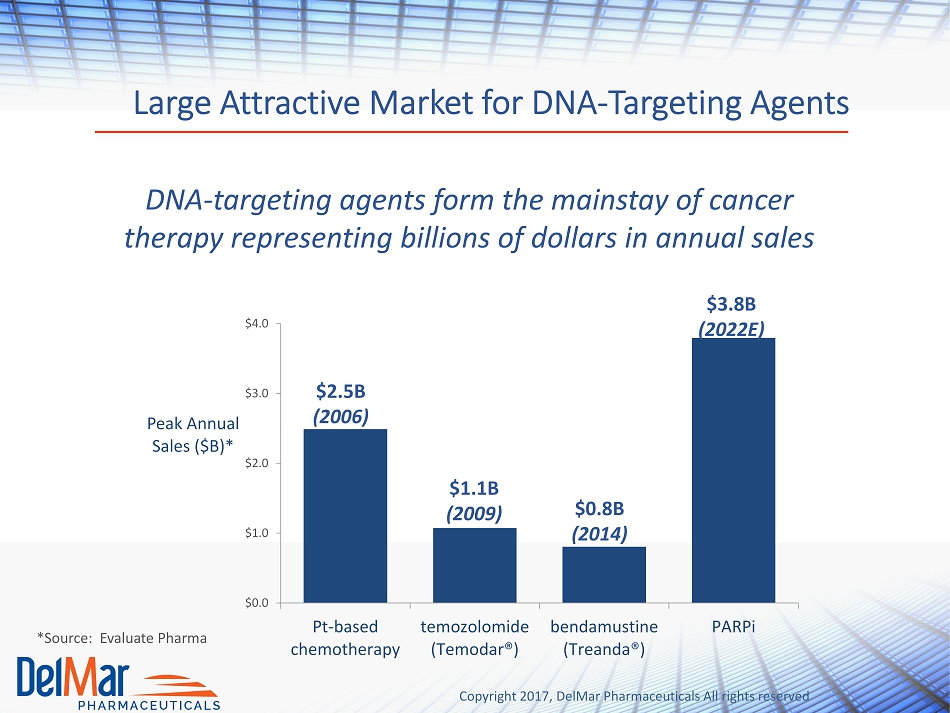
Copyright 2017, DelMar Pharmaceuticals All rights reserved Large Attractive Market for DNA - Targeting Agents DNA - targeting agents form the mainstay of cancer therapy representing billions of dollars in annual sales $0.0 $1.0 $2.0 $3.0 $4.0 Pt-based chemotherapy temozolomide (Temodar®) bendamustine (Treanda®) PARPi Peak Annual Sales ($B)* $2.5B (2006) $1.1B (2009) $0.8B (2014) $3.8B (2022E) *Source: Evaluate Pharma

Copyright 2017, DelMar Pharmaceuticals All rights reserved Selected Recent Highlights • Raised total gross proceeds of $ 19 million via two financings • Initiated pivotal Phase 3 VAL - 083 STAR - 3 clinical trial in refractory GBM • Initiated patient recruitment in Phase 2 front - line MGMT - unmethylated GBM • Received notice of allowance for Phase 1 - 2 VAL - 083 REPROVe trial in platinum - resistant ovarian cancer • Presented research results at numerous peer - reviewed scientific meetings • Expanded network of collaborations with leading academic medical centers • Continued to strengthen intellectual property portfolio • Strengthened our Board of Directors 9

Copyright 2017, DelMar Pharmaceuticals All rights reserved Summary Financial Results 10
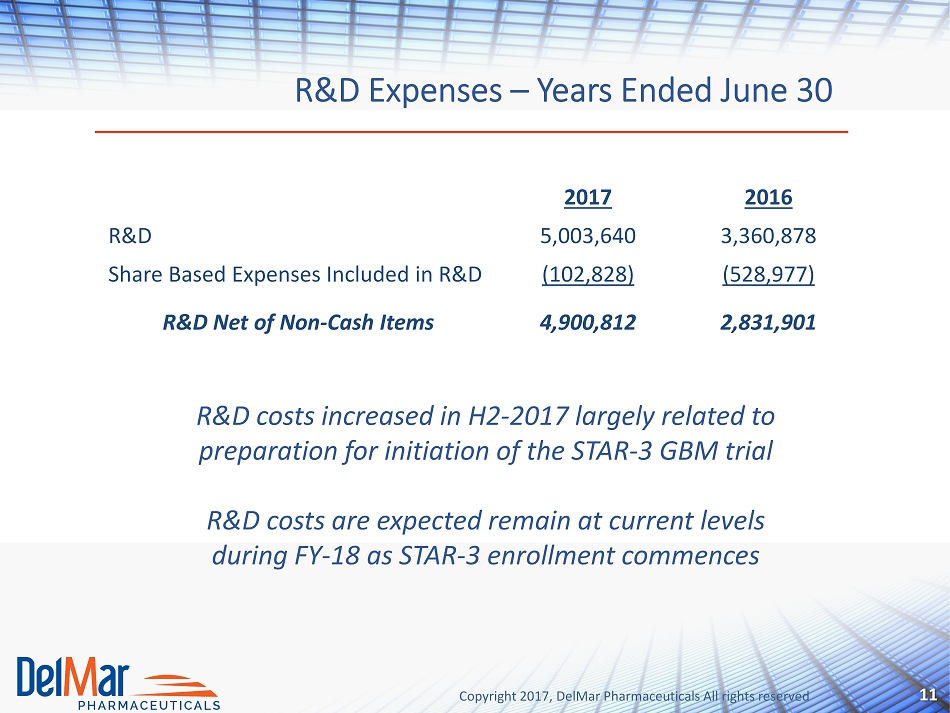
Copyright 2017, DelMar Pharmaceuticals All rights reserved R&D Expenses – Years Ended June 30 11 2017 2016 R&D 5,003,640 3,360,878 Share Based Expenses Included in R&D (102,828) (528,977) R&D Net of Non - Cash Items 4,900,812 2,831,901 R&D costs increased in H2 - 2017 largely related to preparation for initiation of the STAR - 3 GBM trial R&D costs are expected remain at current levels during FY - 18 as STAR - 3 enrollment commences
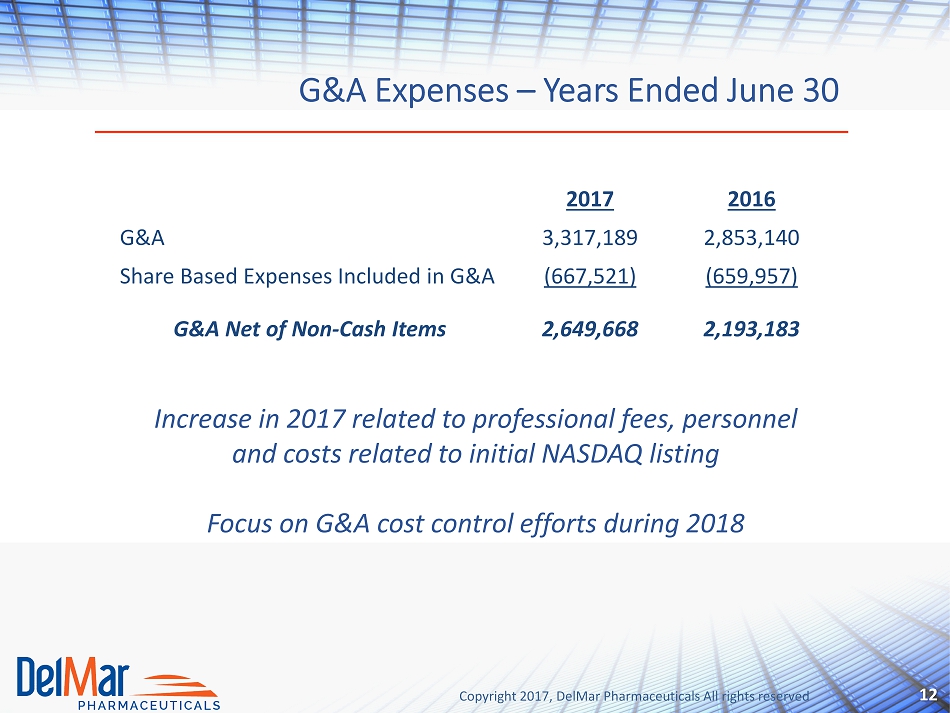
Copyright 2017, DelMar Pharmaceuticals All rights reserved G&A Expenses – Years Ended June 30 12 2017 2016 G&A 3,317,189 2,853,140 Share Based Expenses Included in G&A (667,521) (659,957) G&A Net of Non - Cash Items 2,649,668 2,193,183 Increase in 2017 related to professional fees, personnel and costs related to initial NASDAQ listing Focus on G&A cost control efforts during 2018
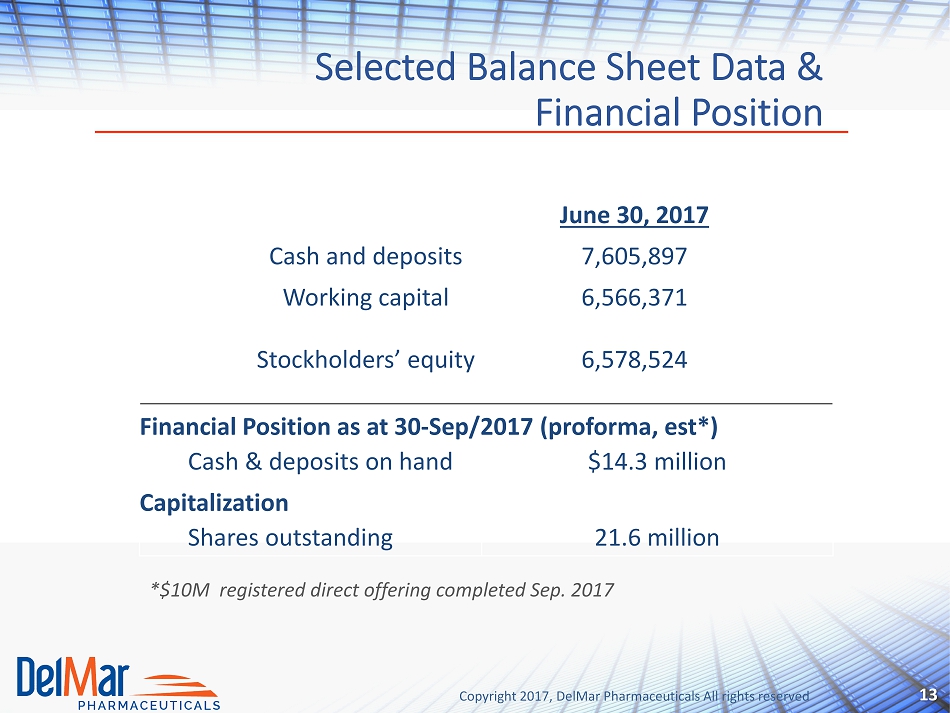
Copyright 2017, DelMar Pharmaceuticals All rights reserved Selected Balance Sheet Data & Financial Position 13 Financial Position as at 30 - Sep/2017 ( proforma , est *) Cash & deposits on hand $14.3 million Capitalization Shares outstanding 21.6 million *$10M registered direct offering completed Sep. 2017 June 30, 2017 Cash and deposits 7,605,897 Working capital 6,566,371 Stockholders’ equity 6,578,524

Copyright 2017, DelMar Pharmaceuticals All rights reserved Update on VAL - 083 Clinical Research Programs 14 • Refractory GBM Pivotal Phase 3 Trial (STAR - 3) • MGMT - unmethylated GBM Two collaborator - supported Phase 2 trials • Ovarian Cancer Phase 1 - 2 VAL - 083 REPROVe trial

Copyright 2017, DelMar Pharmaceuticals All rights reserved First Target Indication: Glioblastoma Multiforme (GBM) • Arises from glial cells which surround neurons in CNS • Approximately 18,000 US and 26,000 EU patients annually* • Current standard of care: 1 st - Surgery , chemotherapy and radiation; 2 nd - Avastin in recurrent GBM ; 3 rd - none approved • Recurrence is nearly universal • Median survival from diagnosis estimated at 15 months** • No new drug therapies increasing median survival have been approved in >30 years * neuropathology - web.org/chapter7/chapter7bGliomas.html#gbm ** Stupp NEJM 2005
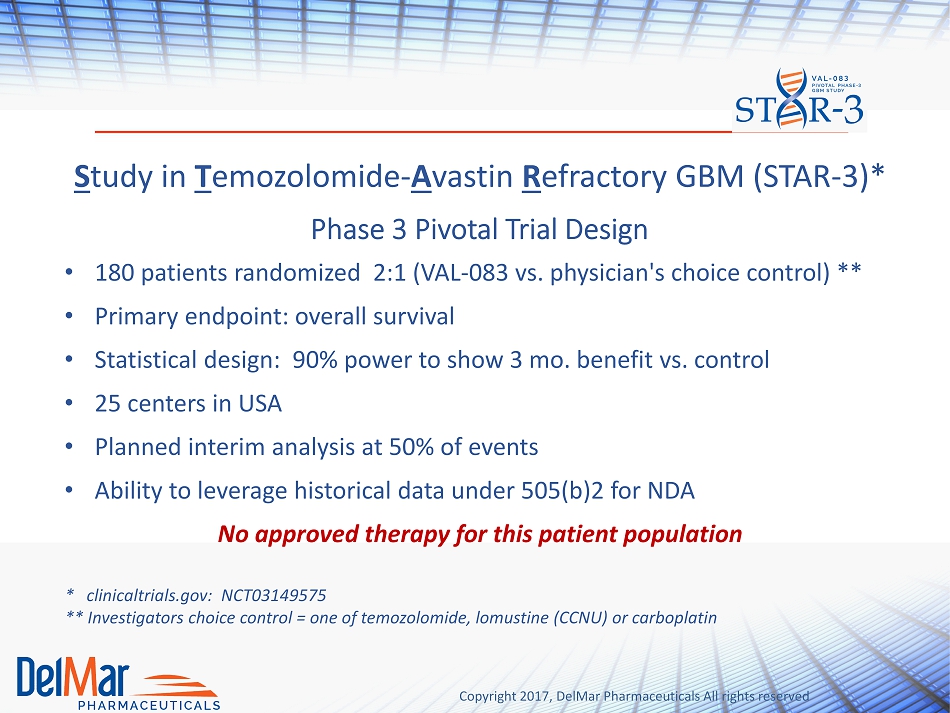
Copyright 2017, DelMar Pharmaceuticals All rights reserved Phase 3 Pivotal Trial Design • 180 patients randomized 2:1 (VAL - 083 vs. physician's choice control) ** • Primary endpoint: overall survival • Statistical design: 90% power to show 3 mo. benefit vs. control • 25 centers in USA • Planned interim analysis at 50% of events • Ability to leverage historical data under 505(b)2 for NDA No approved therapy for this patient population * clinicaltrials.gov : NCT03149575 ** Investigators choice control = one of temozolomide, lomustine (CCNU) or carboplatin S tudy in T emozolomide - A vastin R efractory GBM (STAR - 3)*

Copyright 2017, DelMar Pharmaceuticals All rights reserved • Current Status (30 - Sep/2017) • First site opened recruitment: Aug 2017 • We are on target with our enrollment projections • Study duration: ~ 2 years from patient enrollment • Estimated remaining cost - Final event: ~$9 million - Interim analysis: ~$ 7 million Next update: Society for NeuroOncology (SNO) Annual Meeting (November, 2017) Sites initiated 4 IRB submissions 6 Under review / contract discussions 15 25
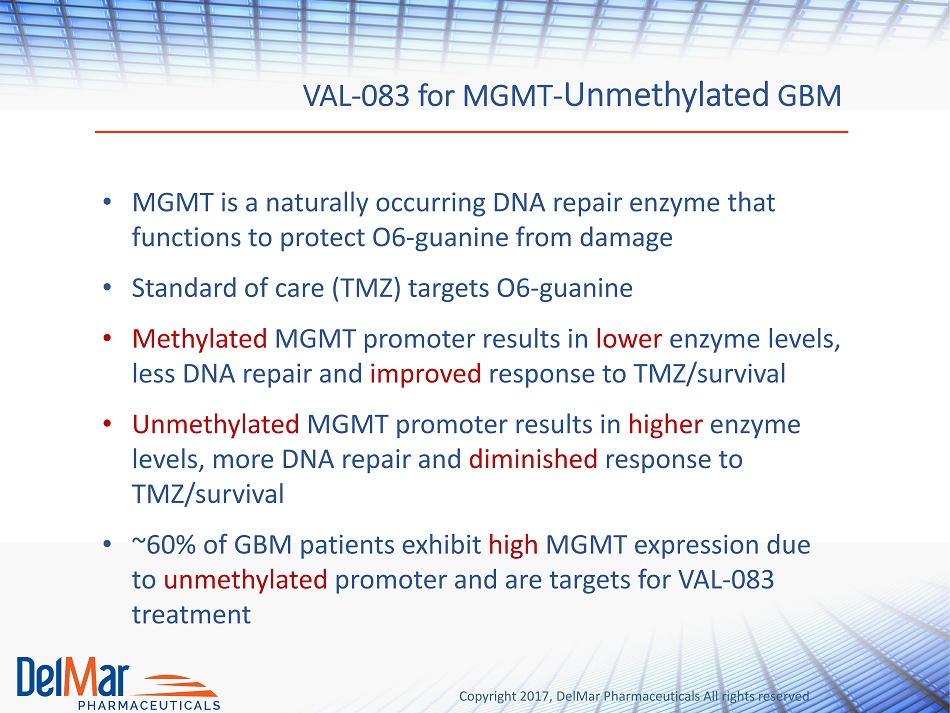
Copyright 2017, DelMar Pharmaceuticals All rights reserved VAL - 083 for MGMT - Unmethylated GBM • MGMT is a naturally occurring DNA repair enzyme that functions to protect O6 - guanine from damage • Standard of care (TMZ) targets O6 - guanine • Methylated MGMT promoter results in lower enzyme levels, less DNA repair and improved response to TMZ/survival • Unmethylated MGMT promoter results in higher enzyme levels, more DNA repair and diminished response to TMZ/survival • ~60% of GBM patients exhibit high MGMT expression due to unmethylated promoter and are targets for VAL - 083 treatment
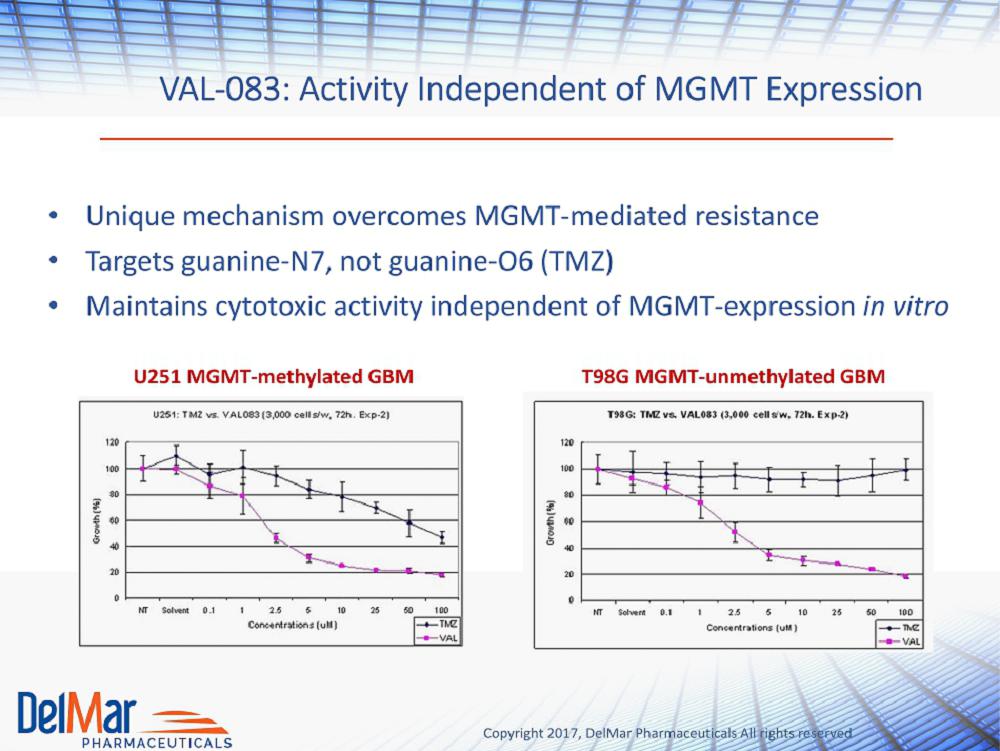
Copyright 2017, DelMar Pharmaceuticals All rights reserved VAL - 083: Activity Independent of MGMT Expression • Unique mechanism overcomes MGMT - mediated resistance • Targets guanine - N7, not guanine - O6 (TMZ) • Maintains cytotoxic activity independent of MGMT - expression in vitro U251 MGMT - methylated GBM T98G MGMT - unmethylated GBM
Copyright 2017, DelMar Pharmaceuticals All rights reserved MGMT - unmethylated GBM Market Opportunity • An estimated 18,000 US and 26,000 EU GBM patients* • Unmethylated MGMT represents ~60% of newly diagnosed GBM, or 26,500 US and EU patients** • 90% of all GBM patients currently treated with TMZ recur within 2 years*** • GBM market is expected to exceed $1.5 billion in 2022**** • VAL - 083 has Orphan Drug Status in US and EU • Two biomarker - driven Phase II studies underway *neuropathology - web.org **Thon OncoTargets (2013) ***Weller J.NeuroOnc (2013) ****Evaluate Pharma
Copyright 2017, DelMar Pharmaceuticals All rights reserved VAL - 083 for 2 nd Line GBM Treatment Recurrent MGMT - unmethylated GBM (UT MD Anderson) • Enroll 48 patients with first recurrence following temozolomide failure • Single arm, Avastin - naïve, confirmed MGMT status • Primary endpoint: Overall Survival • Historical control: Lomustine arm of EORTC 26101 trial OS = 7.1 months (2016) • Data expands cumulative VAL - 083 safety database • Funding support from MD Anderson • Positive outcome provides support for activity in MGMT - unmethylated GBM & strong therapeutic rationale for treatment of recurrent GBM
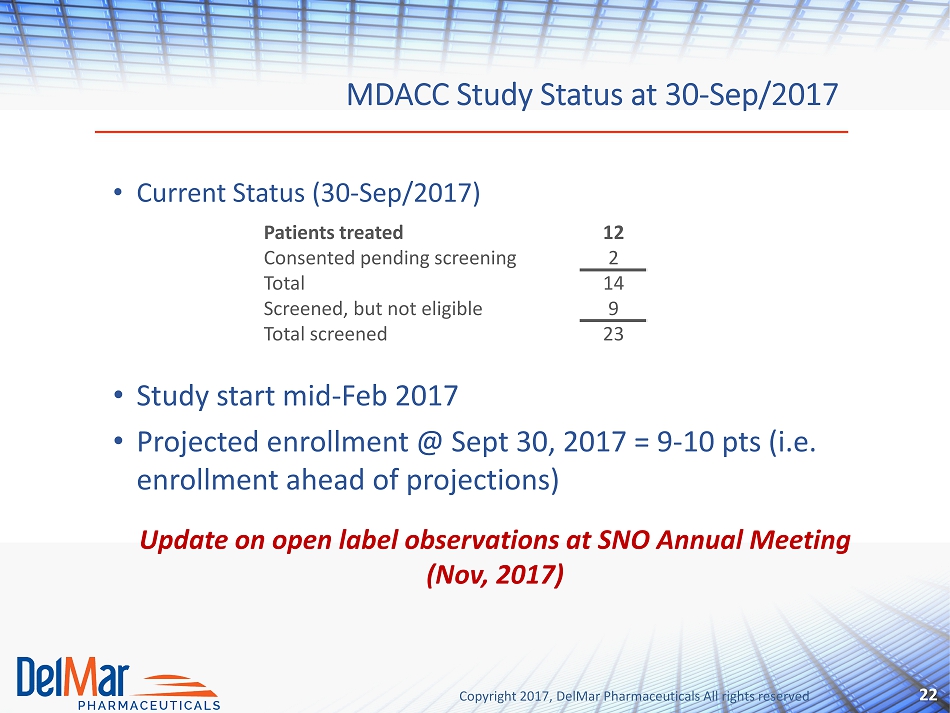
Copyright 2017, DelMar Pharmaceuticals All rights reserved MDACC Study Status at 30 - Sep/2017 22 • Current Status (30 - Sep/2017) • Study start mid - Feb 2017 • Projected enrollment @ Sept 30, 2017 = 9 - 10 pts (i.e. enrollment ahead of projections) Update on open label observations at SNO Annual Meeting ( Nov, 2017) Patients treated 12 Consented pending screening 2 Total 14 Screened, but not eligible 9 Total screened 23

Copyright 2017, DelMar Pharmaceuticals All rights reserved VAL - 083 for 1 st Line GBM Treatment Newly diagnosed MGMT - unmethy lated GBM (International Study) Study initiated September, 2017 • Enroll 30 patients with newly diagnosed MGMT - unmethylated GBM • Single arm, open label, combination VAL - 083 + radiotherapy (XRT) • Primary endpoint: Progression free survival (PFS) - Comparison to historical control : MGMT - unmethylated arm in RTOG 0525 Radiation+ adjuvant TMZ trial (2011) PFS = 5.7 months • Estimated 18 months to top - line efficacy data; 9 months to safety data for combination VAL - 083 + XRT • Funding support from VAL - 083 manufacturing collaboration • Establishes dosing regimen for 1st - line randomized pivotal trial
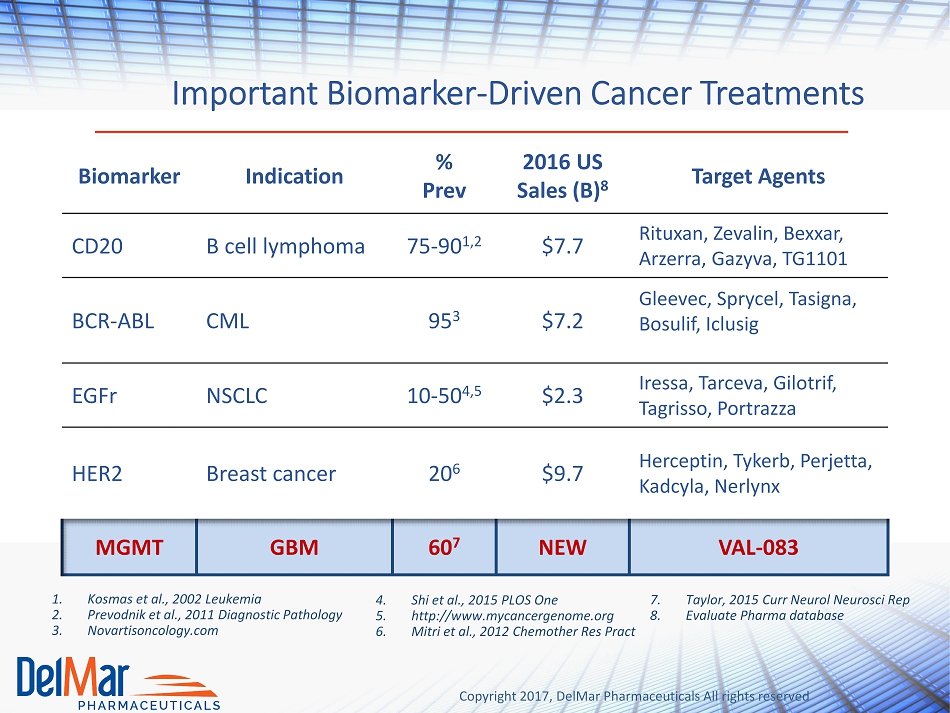
Copyright 2017, DelMar Pharmaceuticals All rights reserved Important Biomarker - Driven Cancer Treatments 1. Kosmas et al., 2002 Leukemia 2. Prevodnik et al., 2011 Diagnostic Pathology 3. Novartisoncology.com 4. Shi et al., 2015 PLOS One 5. http://www.mycancergenome.org 6. Mitri et al., 2012 Chemother Res Pract 7. Taylor, 2015 Curr Neurol Neurosci Rep 8. Evaluate Pharma database Biomarker Indication % Prev 20 16 US Sales (B) 8 Target Agents CD20 B cell lymphoma 75 - 90 1,2 $7.7 Rituxan , Zevalin , Bexxar , Arzerra , Gazyva , TG1101 BCR - ABL CML 95 3 $7.2 Gleevec , Sprycel , Tasigna , Bosulif , Iclusig EGFr NSCLC 10 - 50 4,5 $2.3 Iressa , Tarceva , Gilotrif , Tagrisso , Portrazza HER2 Breast cancer 20 6 $9.7 Herceptin, Tykerb , Perjetta , Kadcyla , Nerlynx MGMT GBM 60 7 NEW VAL - 083

Copyright 2017, DelMar Pharmaceuticals All rights reserved VAL - 083: A Paradigm Shift in the Treatment of GBM • Pivotal STAR - 3 trial streamlines path to market • Potential to overcome chemo - resistance and surpass standard of care TMZ • Overcoming MGMT - mediated treatment failure solves the most significant treatment problem in GBM • Biomarker - driven patient selection using MGMT - methylation • Could create a new survival paradigm for the first time in decades • Addresses >$1 billion market opportunity
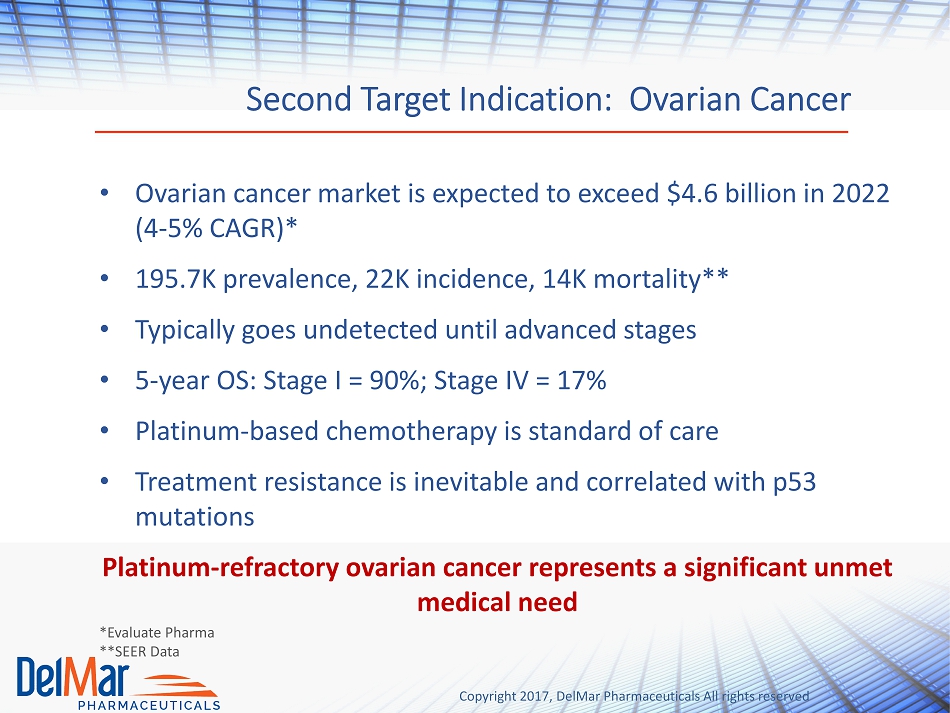
Copyright 2017, DelMar Pharmaceuticals All rights reserved Second Target Indication: Ovarian Cancer • Ovarian cancer market is expected to exceed $4.6 billion in 2022 ( 4 - 5% CAGR)* • 195.7K prevalence, 22K incidence, 14K mortality** • Typically goes undetected until advanced stages • 5 - year OS: Stage I = 90%; Stage IV = 17% • Platinum - based chemotherapy is standard of care • Treatment resistance is inevitable and correlated with p53 mutations Platinum - refractory ovarian cancer represents a significant unmet medical need *Evaluate Pharma **SEER Data
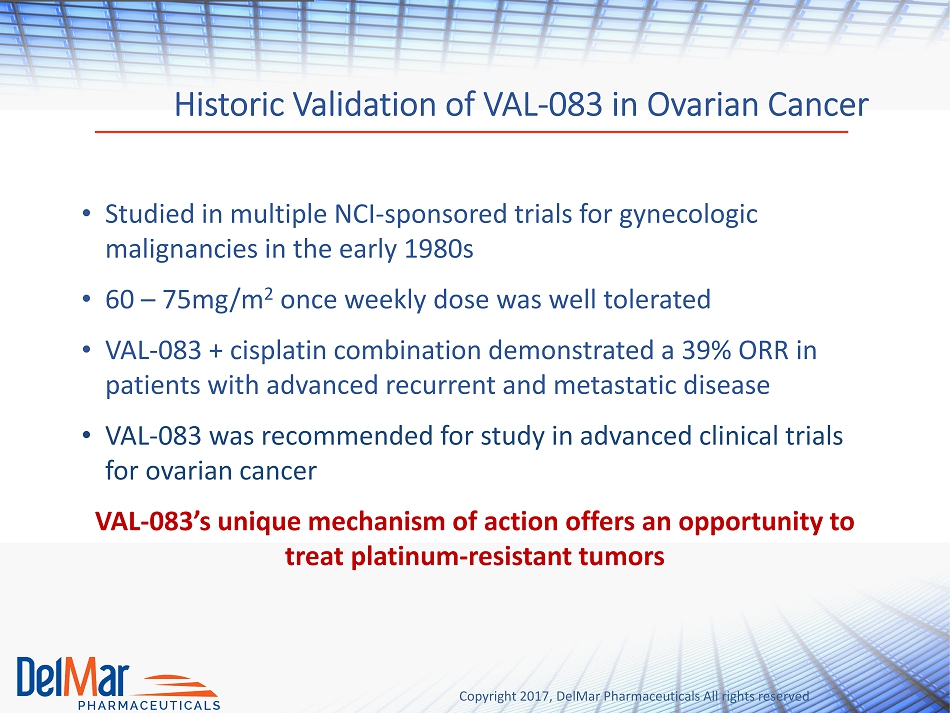
Copyright 2017, DelMar Pharmaceuticals All rights reserved Historic Validation of VAL - 083 in Ovarian Cancer • Studied in multiple NCI - sponsored trials for gynecologic malignancies in the early 1980s • 60 – 75mg/m 2 once weekly dose was well tolerated • VAL - 083 + cisplatin combination demonstrated a 39% ORR in patients with advanced recurrent and metastatic disease • VAL - 083 was recommended for study in advanced clinical trials for ovarian cancer VAL - 083’s unique mechanism of action offers an opportunity to treat platinum - resistant tumors
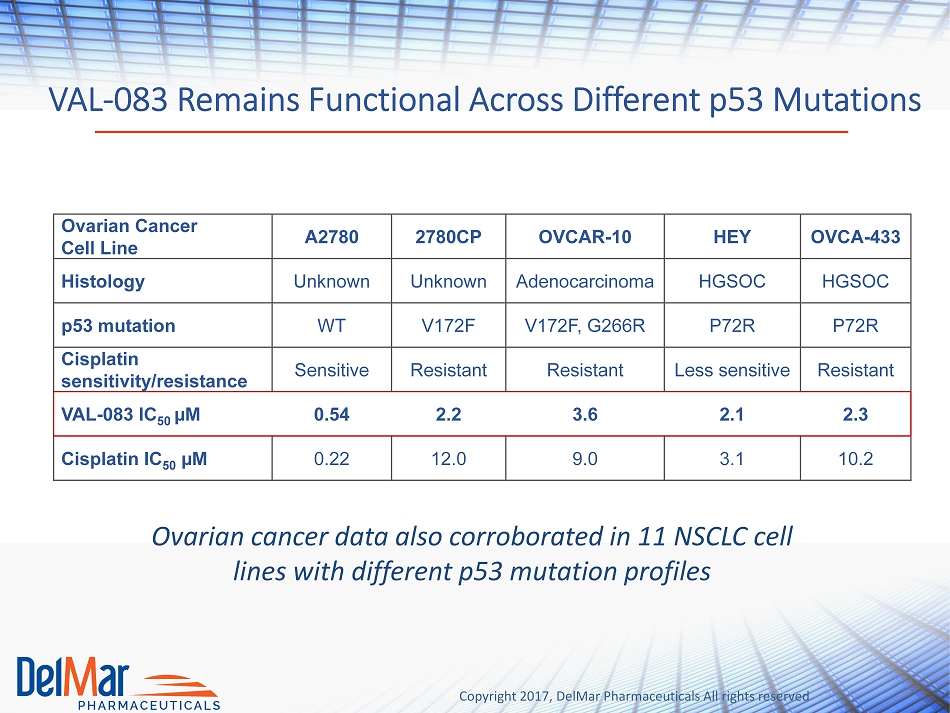
Copyright 2017, DelMar Pharmaceuticals All rights reserved VAL - 083 Remains Functional Across Different p53 Mutations Ovarian cancer data also corroborated in 11 NSCLC cell lines with different p53 mutation profiles Ovarian Cancer Cell Line A2780 2780CP OVCAR - 10 HEY OVCA - 433 Histology Unknown Unknown Adenocarcinoma HGSOC HGSOC p 53 mutation WT V172F V172F, G266R P72R P72R Cisplatin sensitivity/resistance Sensitive Resistant Resistant Less sensitive Resistant VAL - 083 IC 50 µM 0.54 2.2 3.6 2.1 2.3 Cisplatin IC 50 µM 0.22 12.0 9.0 3.1 10.2

Copyright 2017, DelMar Pharmaceuticals All rights reserved Clinical Development Strategy in Ovarian Cancer • PI: Dr. Bradley Monk (led successful Tesaro Phase 3 PARPi ovarian trial ) • Enroll up to 24 patients to establish proof of concept ( PoC ) • Primary endpoint: Overall response rate vs. historical control • If successful, expand study to 60 patients in Phase 2 • Positive results used to seek accelerated approval or to guide pivotal trial design CURRENT STATUS • IND allowance by FDA on Sept. 19, 2017 • Contract/budget discussions with clinical sites underway • Top - line ( PoC ) results: ~18 months from initiation Phase 1/2 trial in Re current P latinum - R esistant Ov arian Cancer ( REPROVe Trial)

Copyright 2017, DelMar Pharmaceuticals All rights reserved Efficient VAL - 083 Manufacturing • Ease of manufacture: chemical synthesis is fewer than five steps • Producing lyophilized drug product for iv administration • European commercial - scale GMP manufacturing partner secured for Phase 3 trial and marketed drug supply • Stability of API material is readily managed • Small - molecule COGS are expected to drive attractive pharmaceutical margins

Copyright 2017, DelMar Pharmaceuticals All rights reserved Strong Intellectual Property Protection • 14 separate patent families covering broad claims • Claims include use, manufacturing, analytical, mechanism of action, and composition • 8 US patents and 8 international patents issued to date • Issued claims provide US patent protection until 2033 • >100 patent filings + 4 provisional applications pending on a global basis • VAL - 083 granted Orphan Drug Designation in the US & EU

Copyright 2017, DelMar Pharmaceuticals All rights reserved Board of Directors 32 Erich Mohr , PhD, R - Psych Independent Chair Founder , Chairman & CEO: MedGenesis Therapeutix ; Co - founder : CroMedica Jeffrey Bacha , BSc MBA President & Chief Executive Officer John K. Bell , CPA Chairman, Onbelay Capital; Board of Royal Canadian Mint; Director, Canopy Growth Corp Dennis Brown , PhD Chief Scientific Officer Founder , Chemgenex and Matrix Pharmaceuticals Lynda Cranston , BScN , MScN , ICD.D Former CEO of the B.C. Provincial Health Services Authority Robert J. Toth , MBA Former Senior Vice President and Biotechnology Analyst, Prudential Securities Saiid Zarrabian Former Chairman Director of La Jolla Pharmaceutical Company
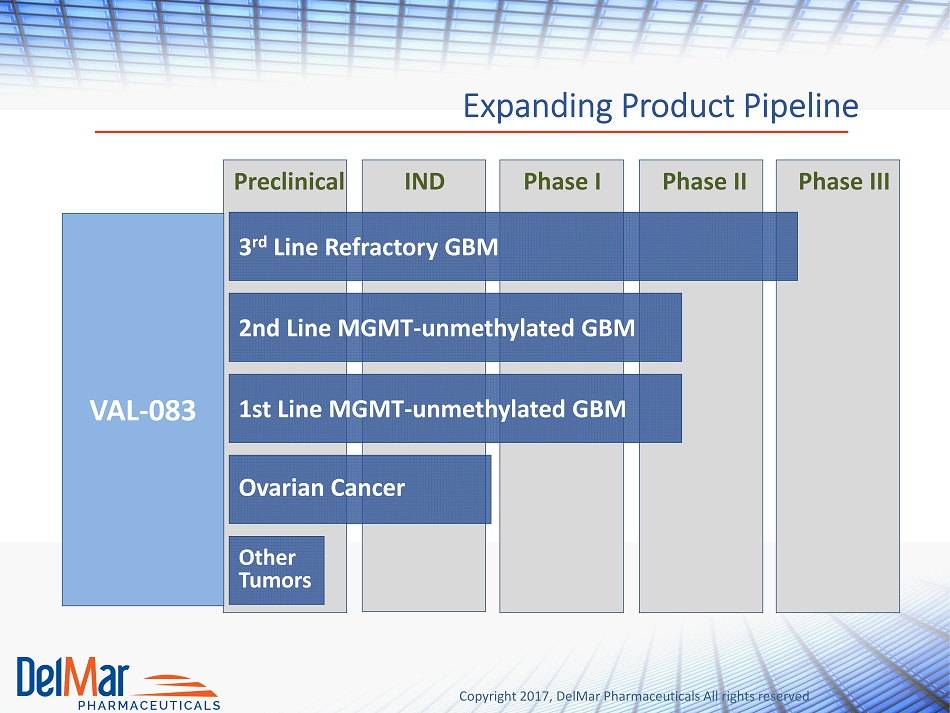
Copyright 2017, DelMar Pharmaceuticals All rights reserved Expanding Product Pipeline Preclinical IND Phase I Phase II Phase III VAL - 083 Other Tumors Ovarian Cancer 1st Line MGMT - unmethylated GBM 2nd Line MGMT - unmethylated GBM 3 rd Line Refractory GBM

Copyright 2017, DelMar Pharmaceuticals All rights reserved Investment Highlights • Newly strengthened balance sheet positions us to advance multiple clinical programs • Lead program in a single pivotal phase 3 clinical trial — GBM with expected interim read - out in early 2019 • Three additional clinical studies including one in ovarian cancer • Potential for accelerated FDA approval timelines • Targeting large market opportunities with significant unmet medical needs • Orphan drug designation in multiple US and EU indications • Strong intellectual property protection • Leveraging 25 years of NCI clinical work and data

Copyright 2017, DelMar Pharmaceuticals All rights reserved