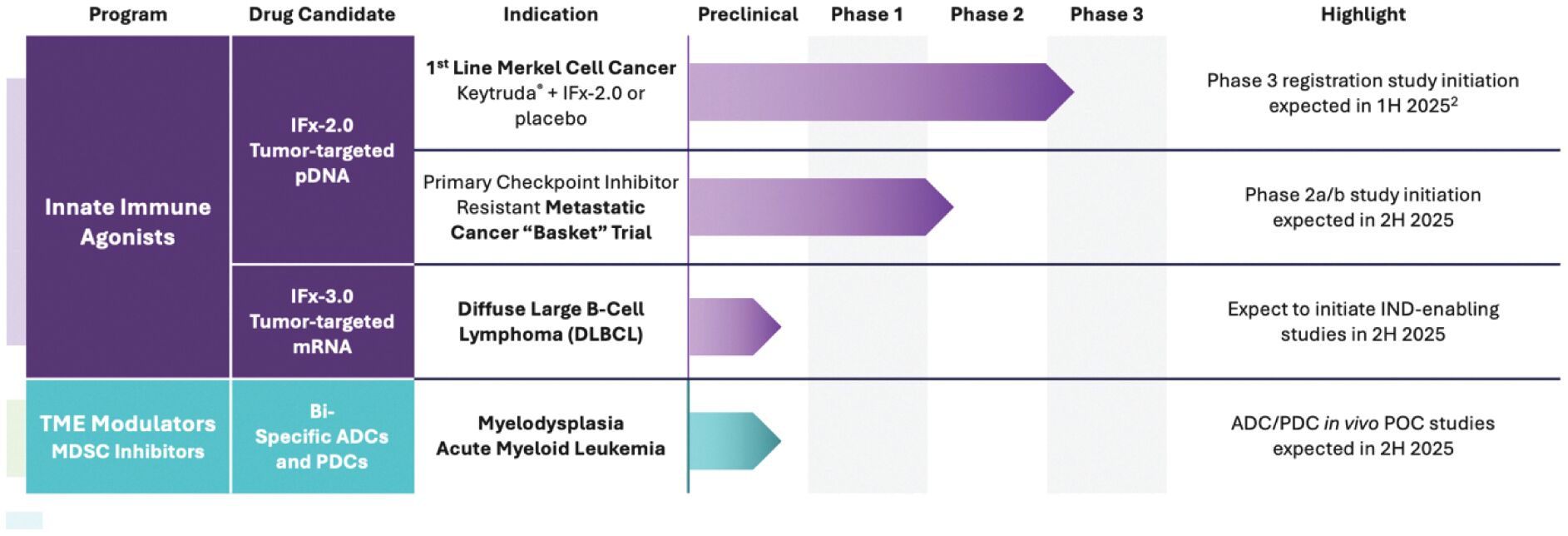
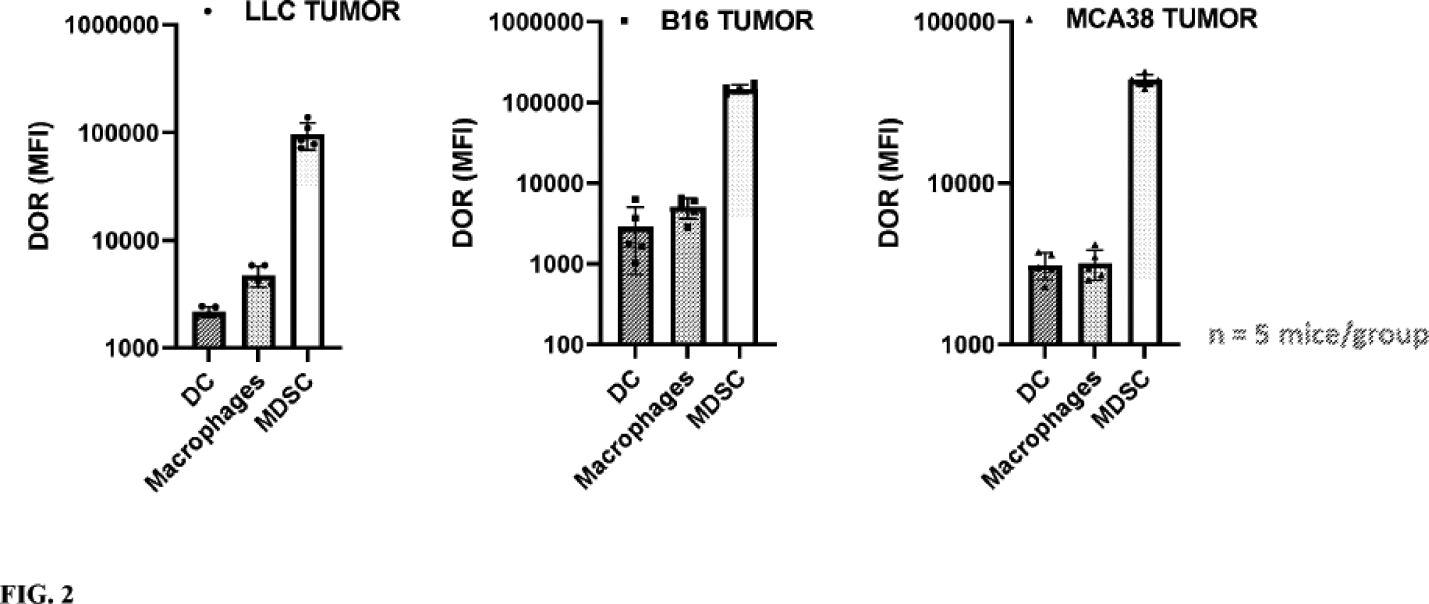
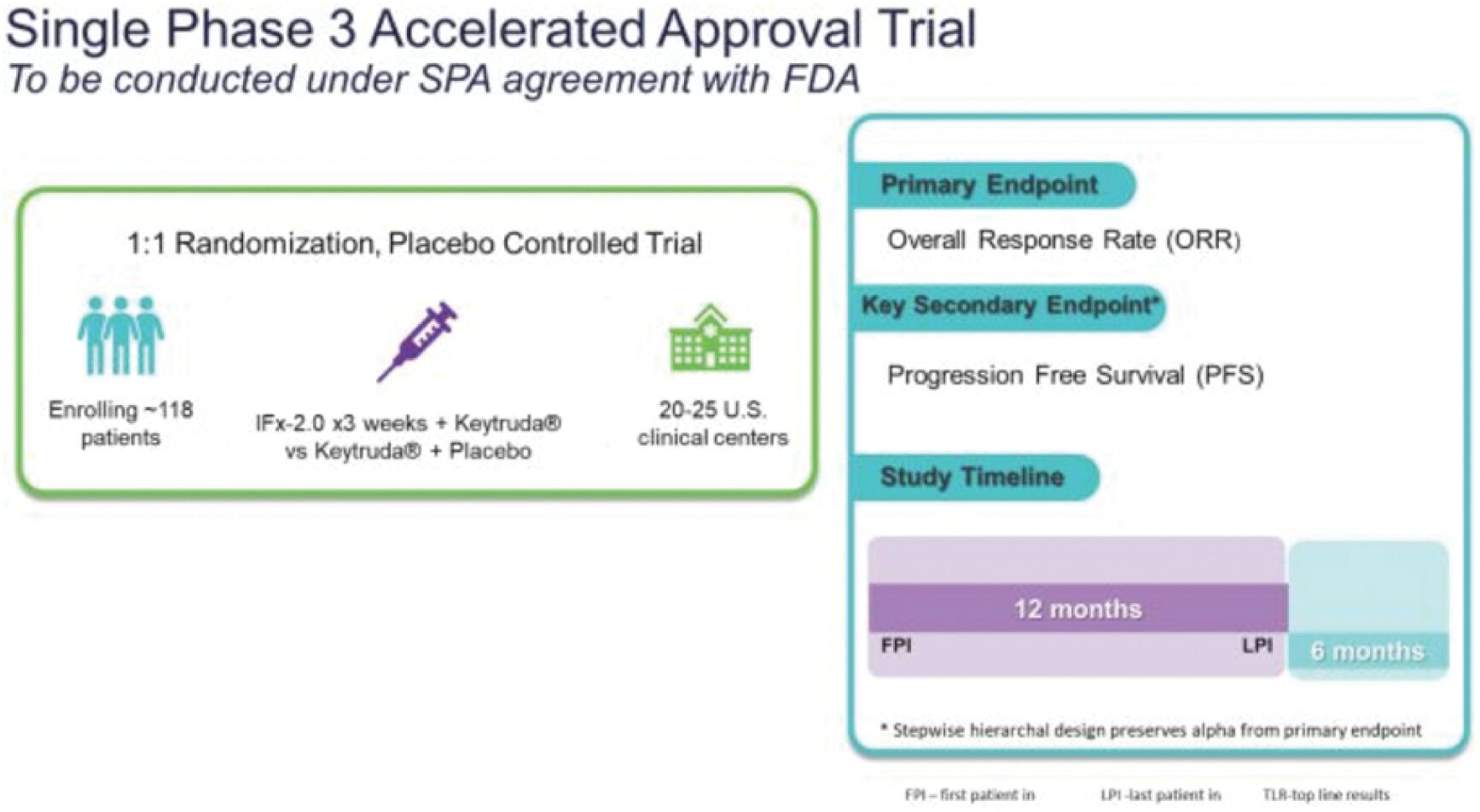
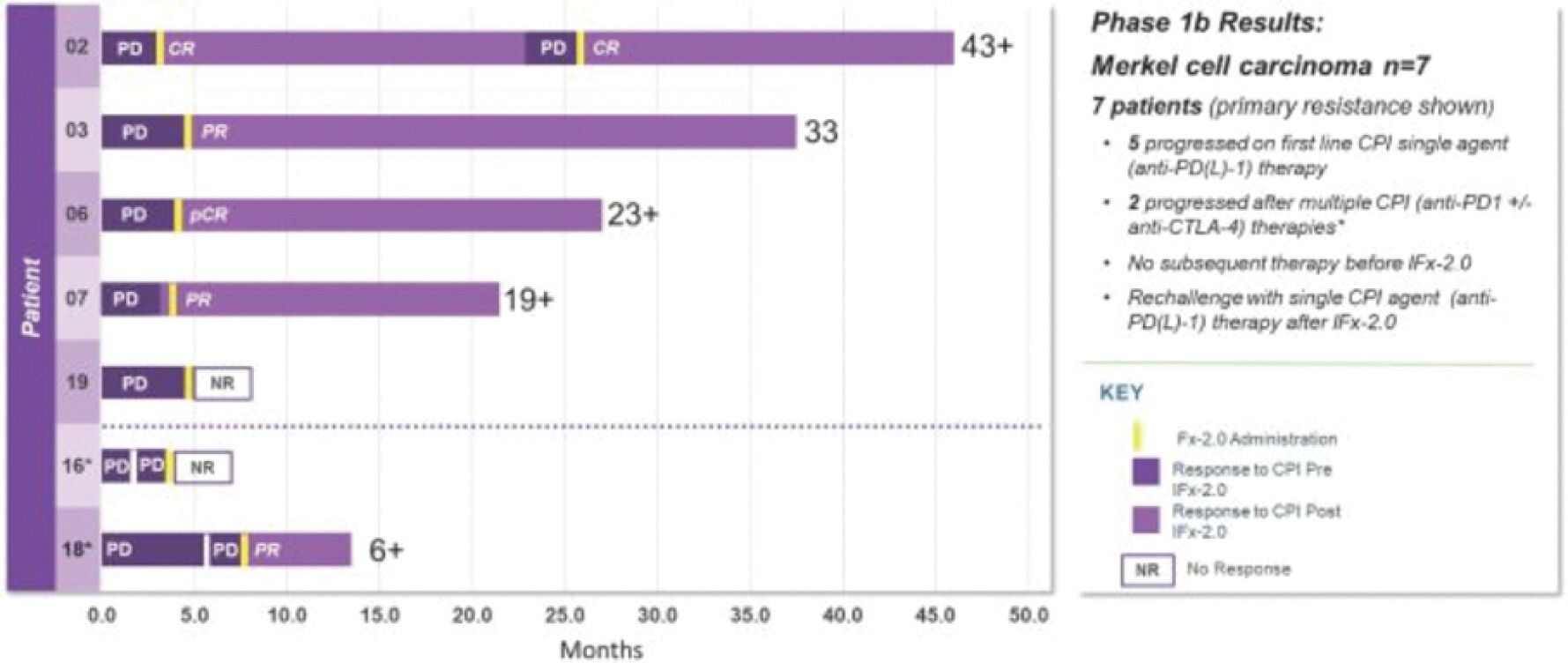
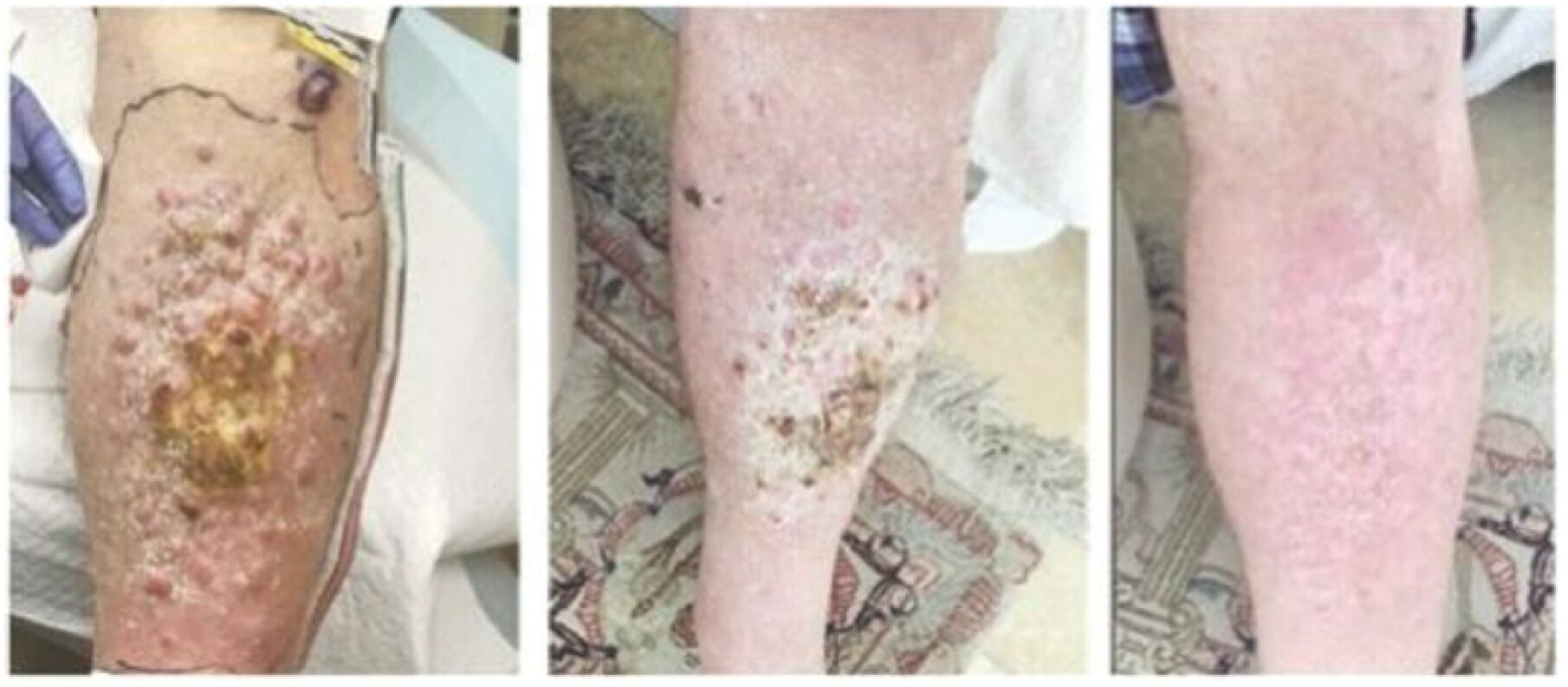
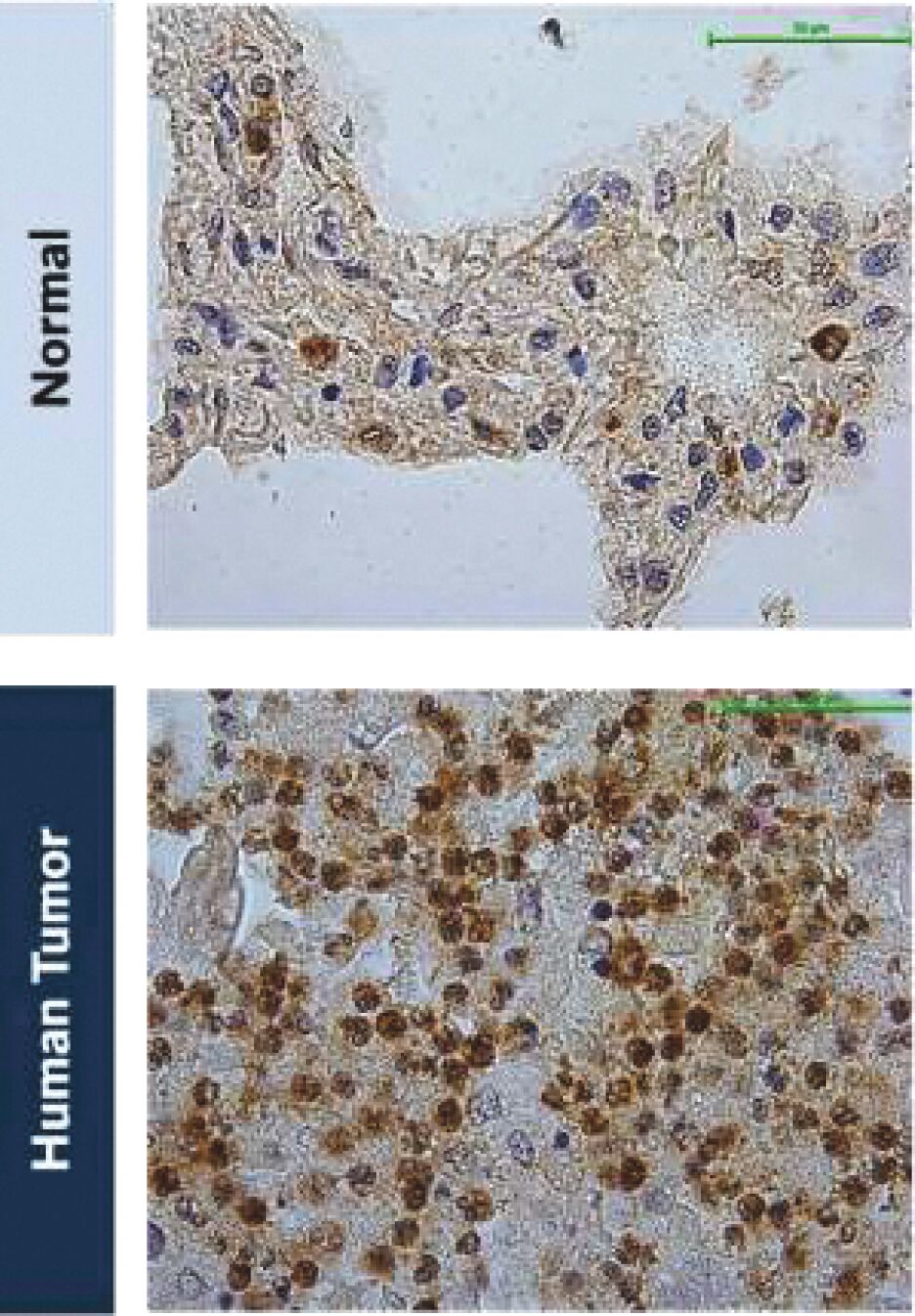
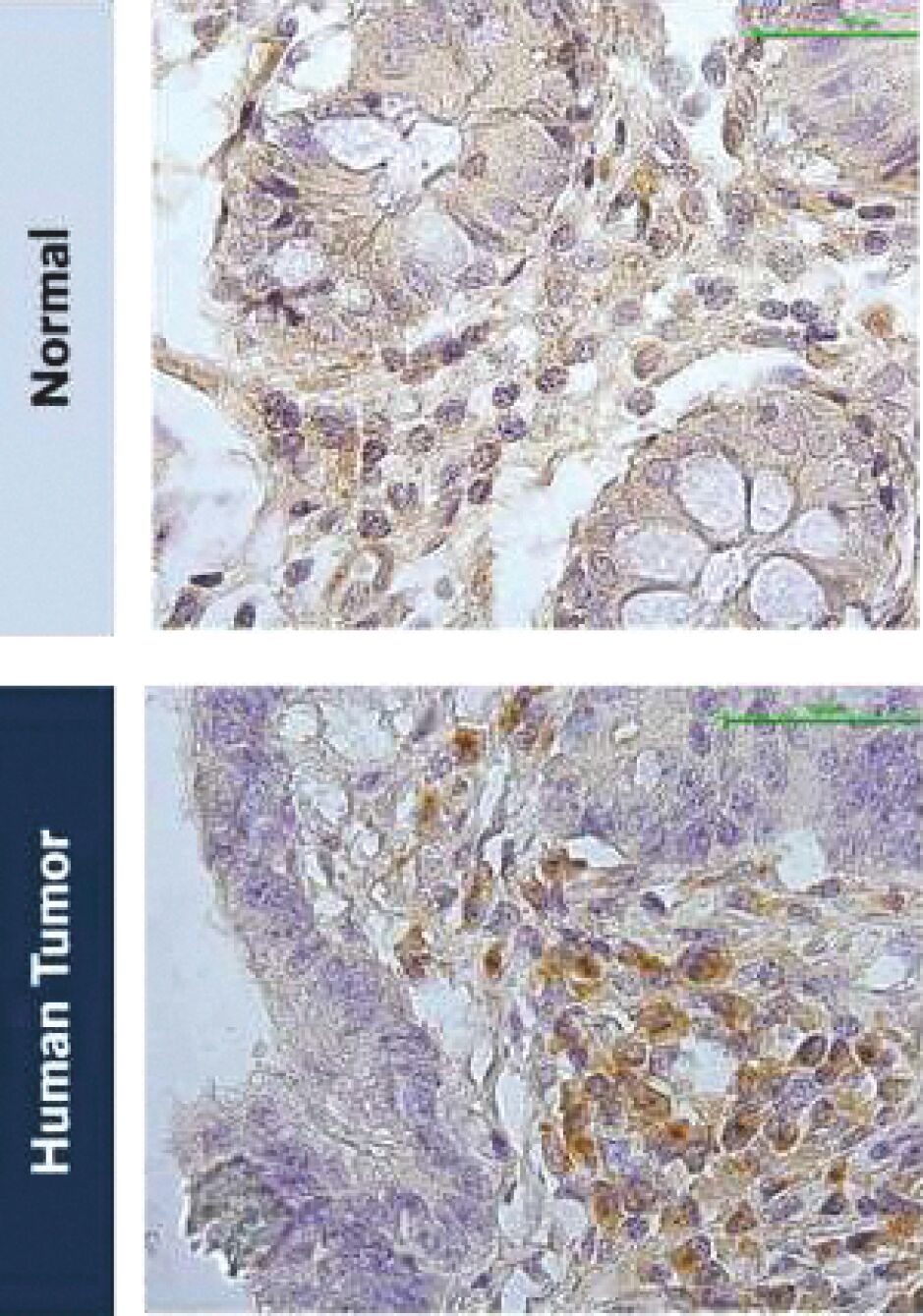
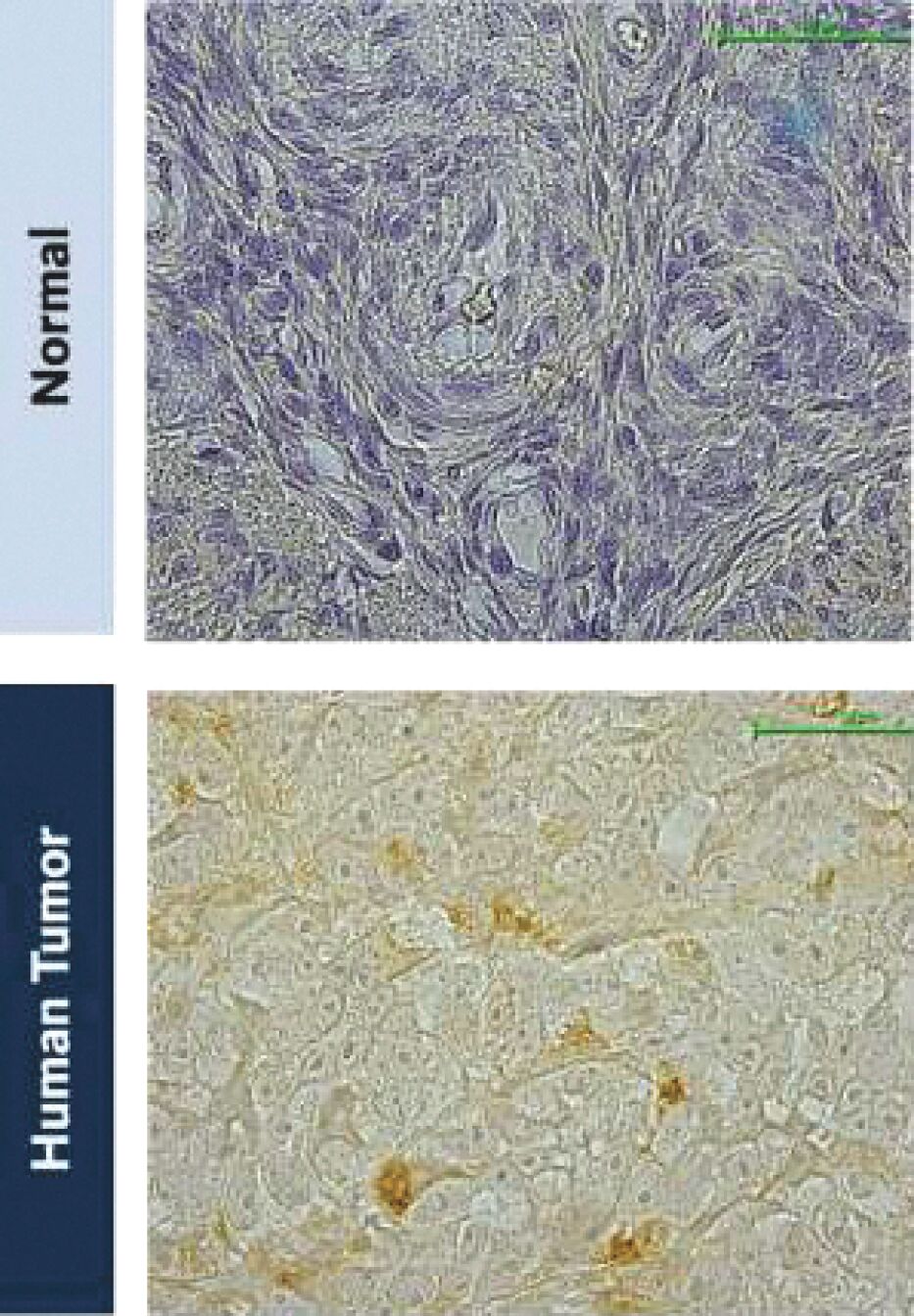
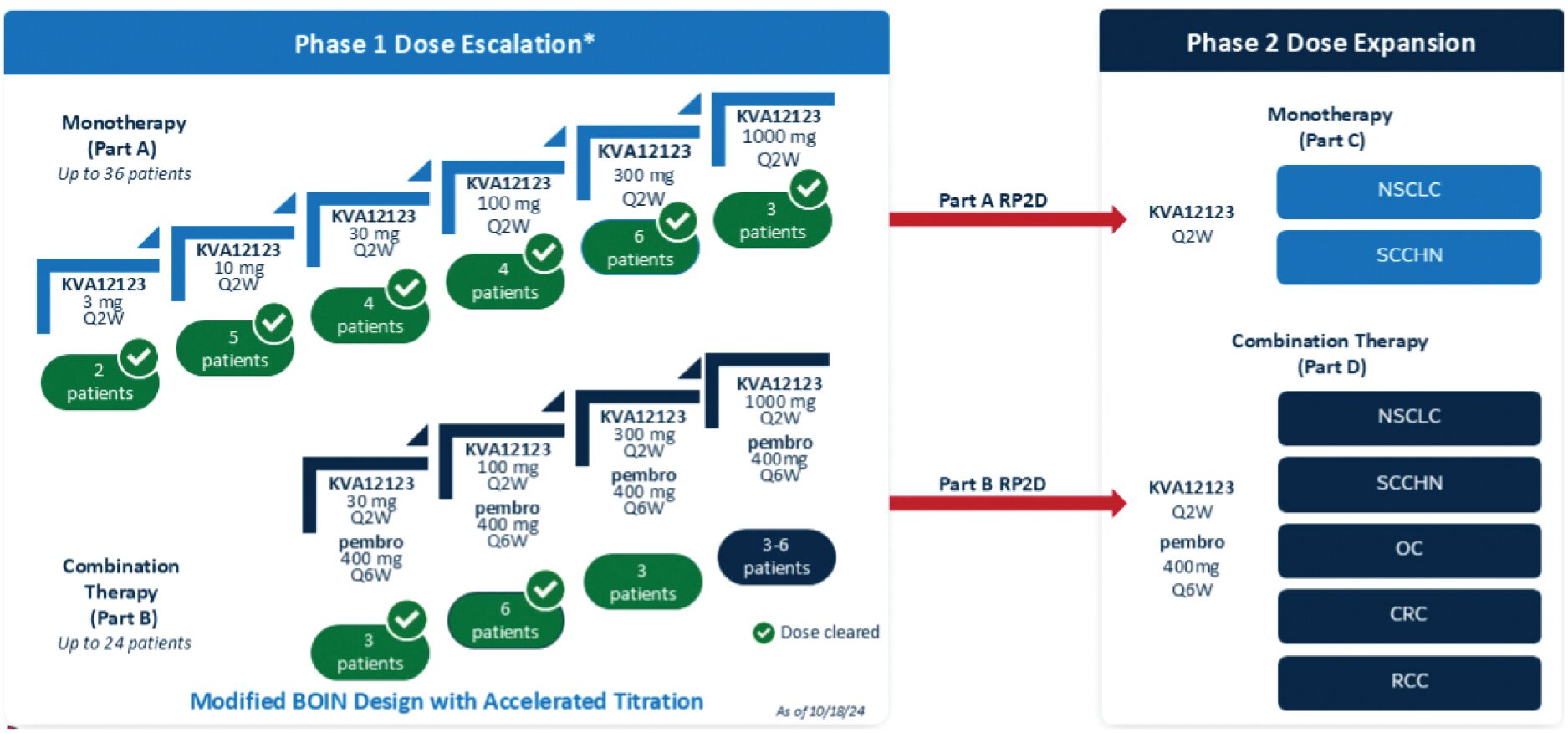
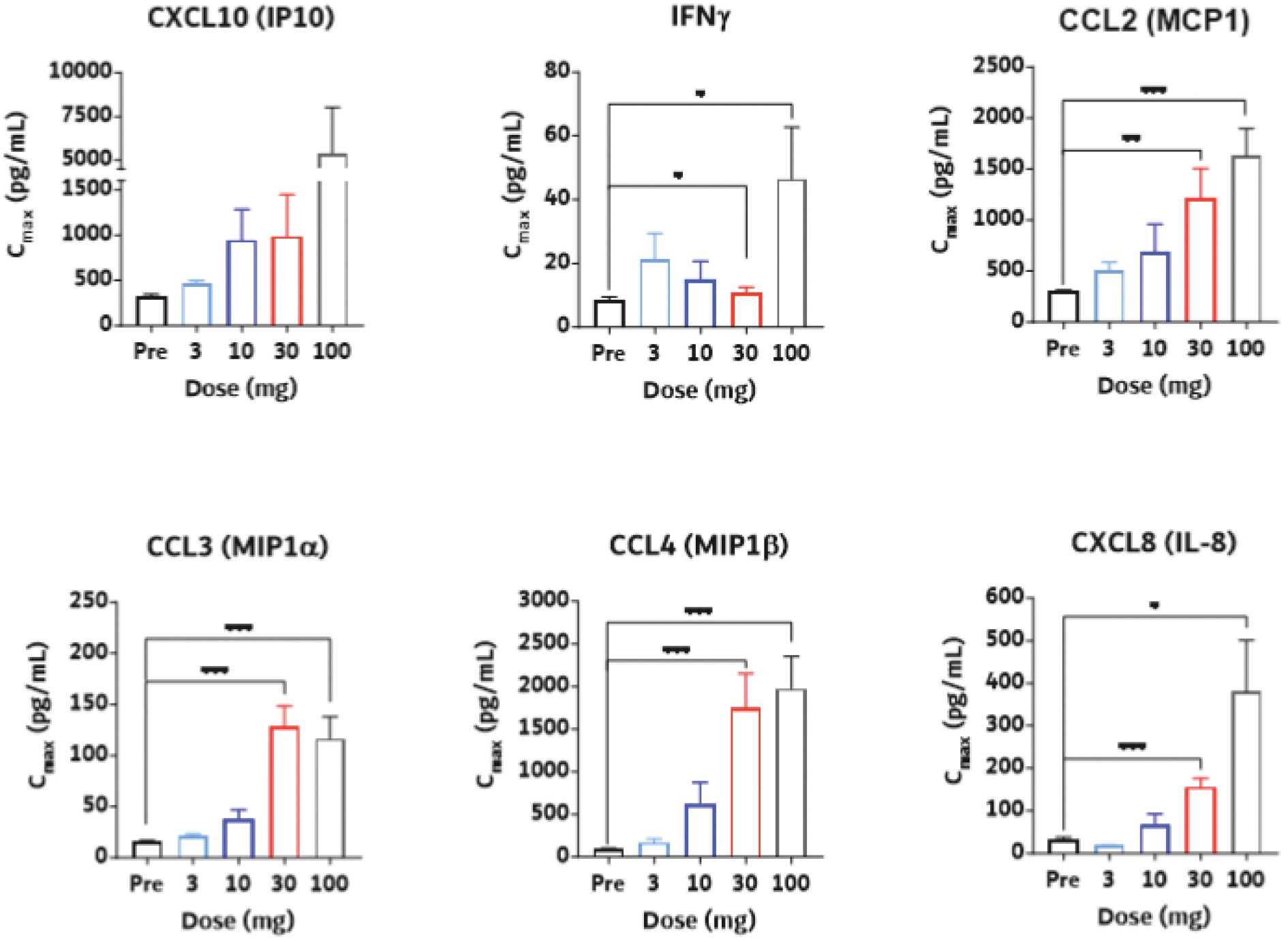
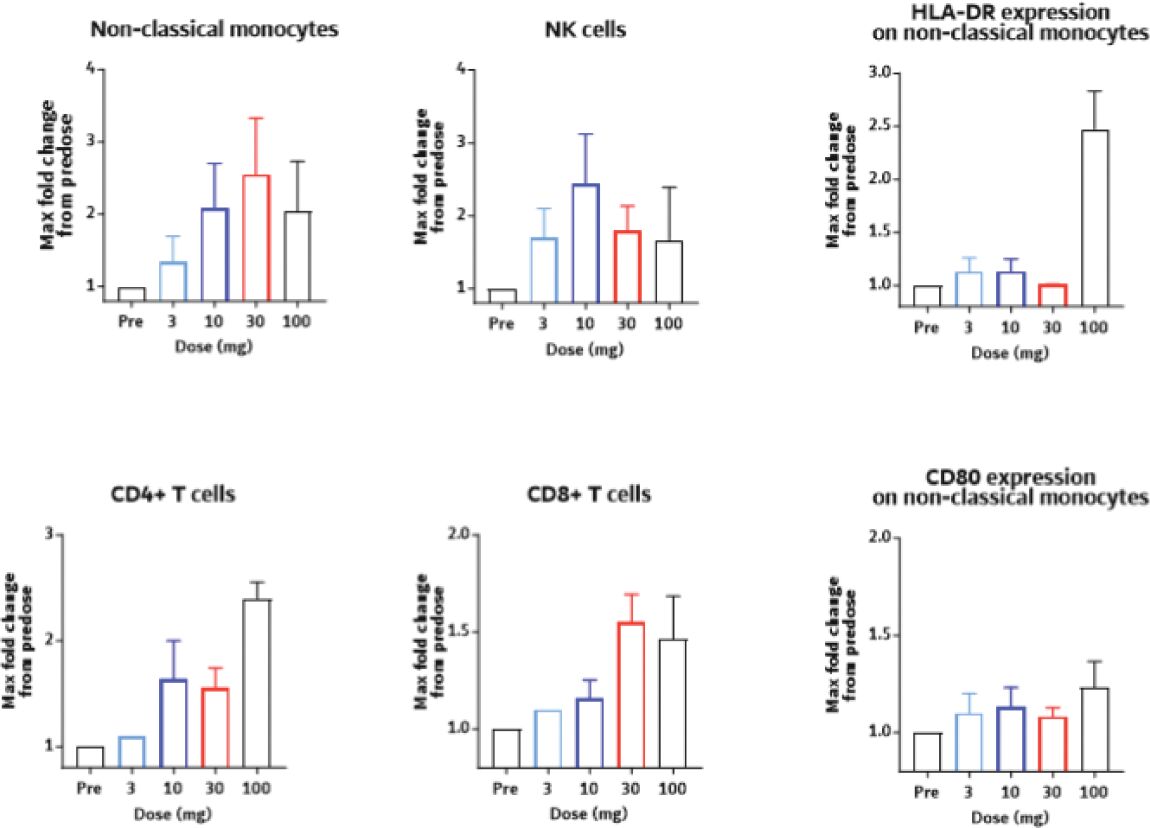
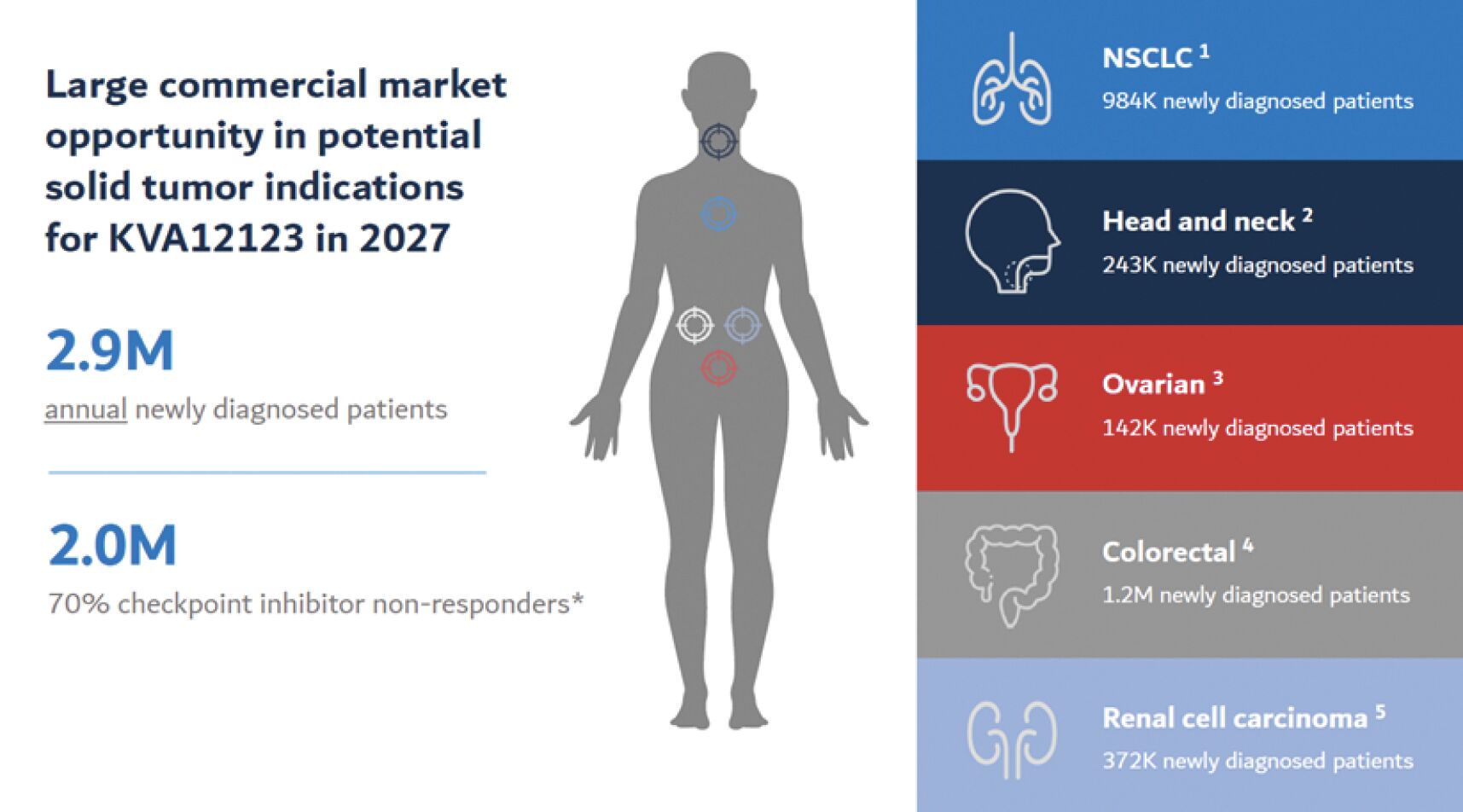
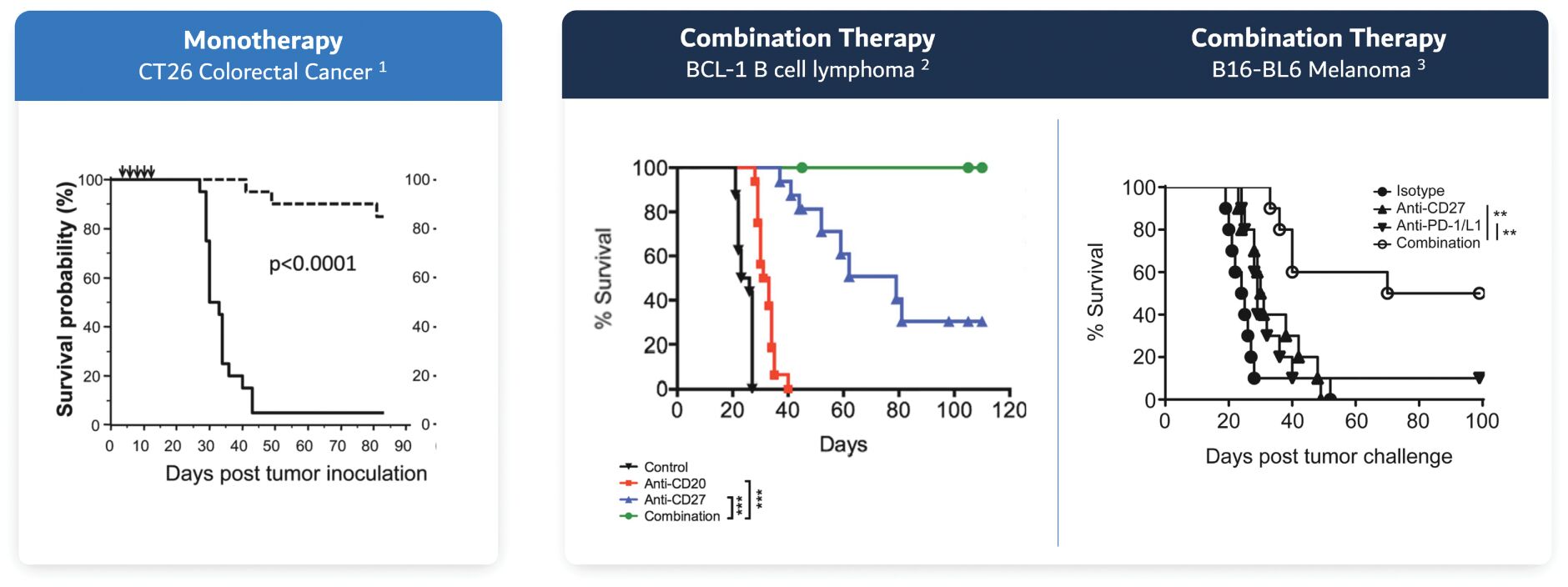
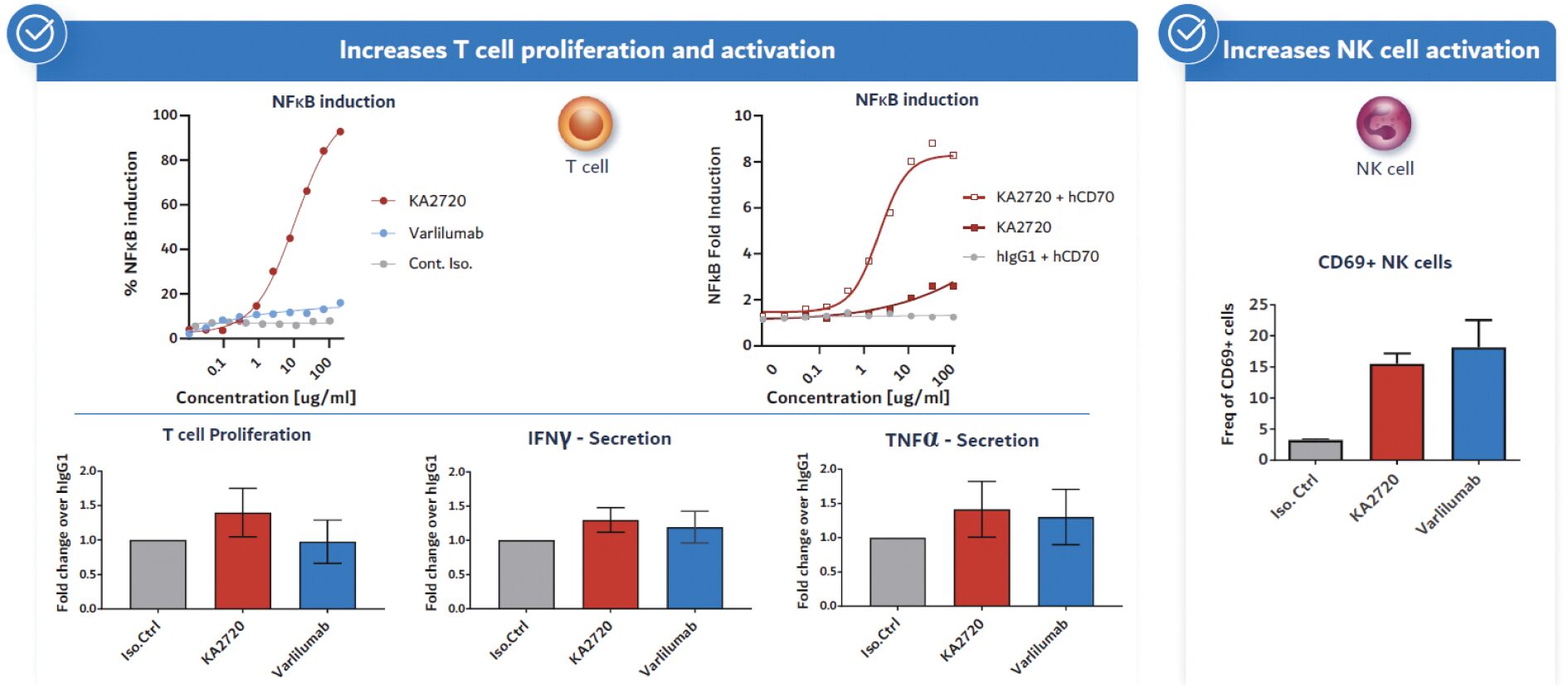
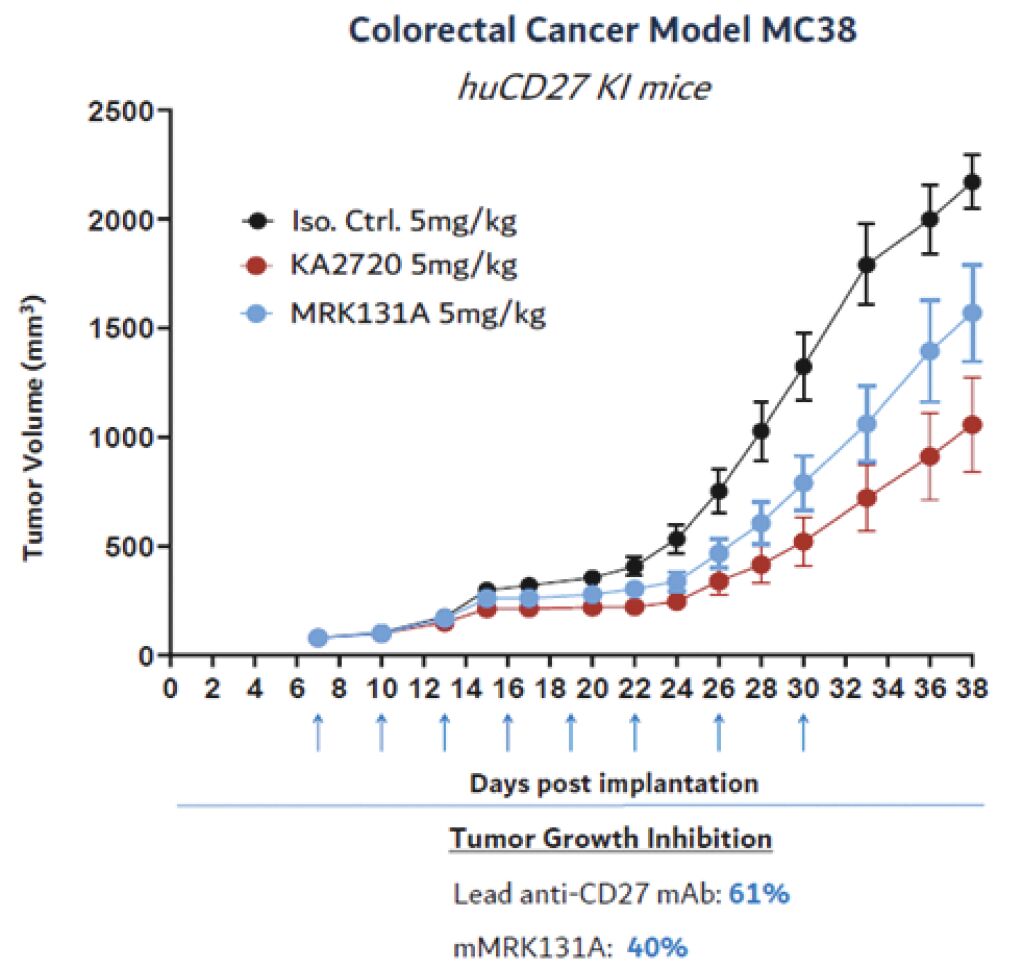
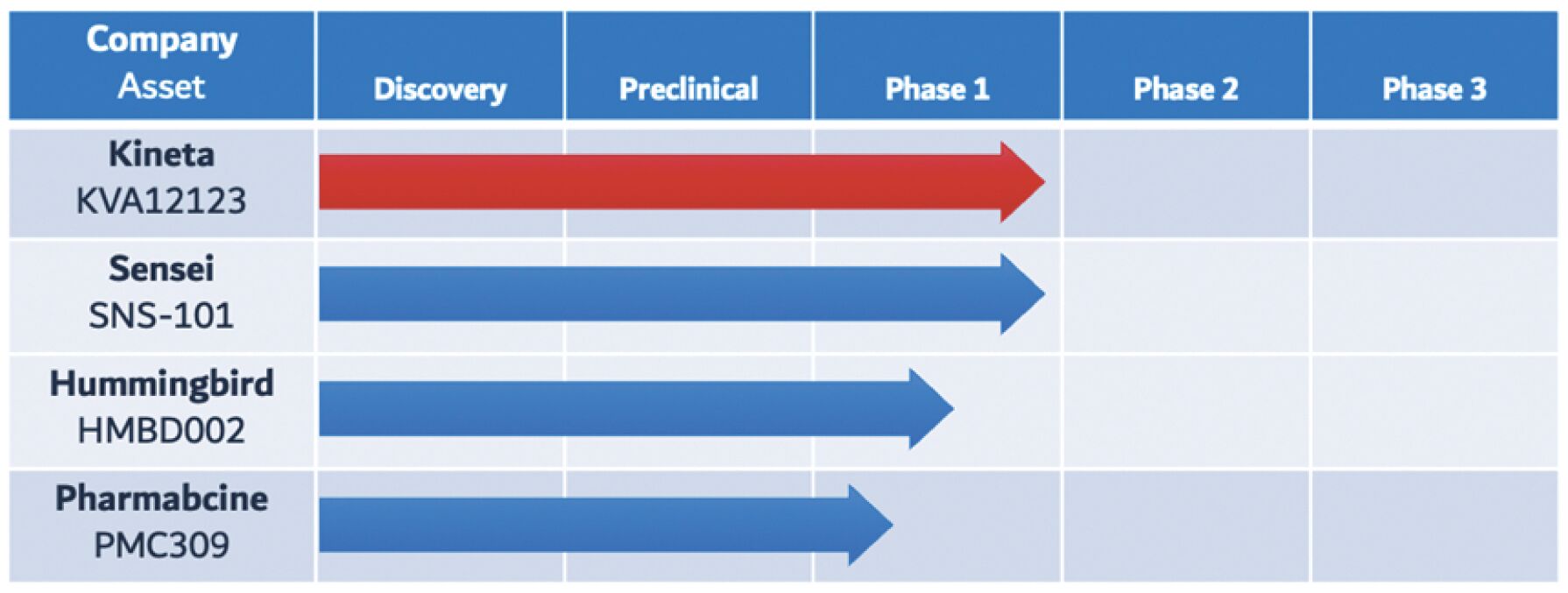
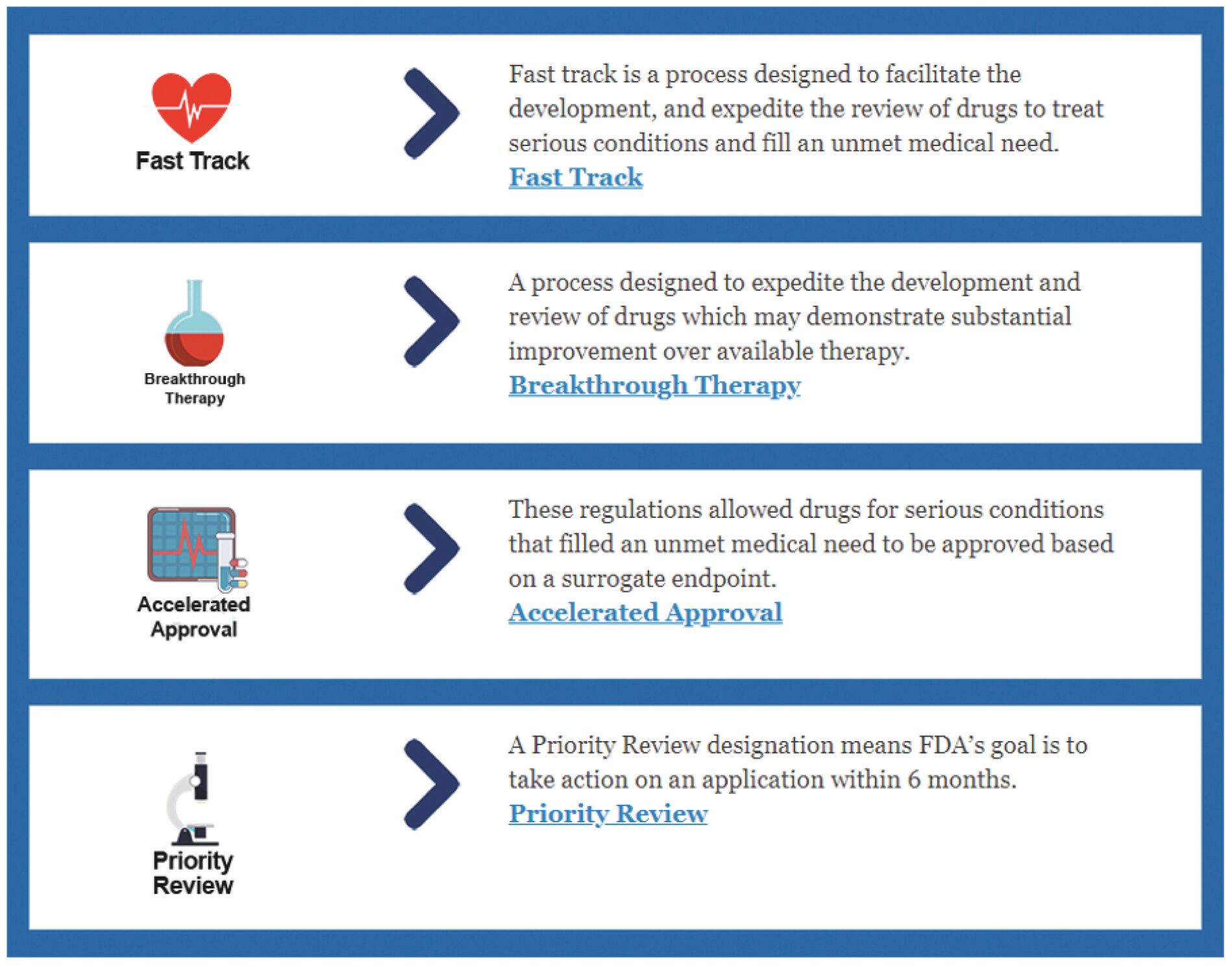
The information in this joint proxy statement/prospectus is not complete and may be changed. A registration statement relating to the securities described in this joint proxy statement/prospectus has been filed with the U.S. Securities and Exchange Commission. These securities may not be issued until the registration statement filed with the U.S. Securities and Exchange Commission is effective. This joint proxy statement/prospectus does not constitute an offer to sell or the solicitation of offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
PRELIMINARY – SUBJECT TO COMPLETION, DATED FEBRUARY 7, 2025

|

|
MERGER AGREEMENT PROPOSAL – YOUR VOTE IS VERY IMPORTANT
Dear TuHURA Biosciences, Inc. Stockholders and Kineta, Inc. Stockholders:
On December 11, 2024, TuHURA Biosciences, Inc., a Nevada corporation (“TuHURA”), Hura Merger Sub I, Inc., a Delaware corporation and a direct wholly-owned subsidiary of TuHURA (“Merger Sub I”), Hura Merger Sub II, LLC, a Delaware limited liability company and direct wholly-owned subsidiary of TuHURA (“Merger Sub II,” and together with Merger Sub I, the “Merger Subs”), Kineta, Inc., a Delaware corporation (the “Company” or “Kineta”) and Craig Philips, solely in his capacity as the representative, agent and attorney-in-fact of the stockholders of Kineta entered into an agreement and plan of merger, as it may be amended from time to time (the “Merger Agreement”), pursuant to which TuHURA agreed to acquire the Company in a cash and stock transaction through mergers of affiliates of TuHURA with and into Kineta with Kineta surviving the mergers (the “Mergers”). TuHURA stockholders are cordially invited to attend a special meeting of stockholders of TuHURA to be held on [●], virtually via the Internet at [●], at [●], Eastern Time, including any adjournments or postponements thereof (the “TuHURA special meeting”) to consider and vote on a proposal to amend TuHURA’s Articles of Incorporation, as amended, to increase the number of authorized shares of common stock, $0.001 par value per share, of TuHURA (“TuHURA Common Stock”) from 75 million shares to 200 million shares (the “Authorized Share Increase Proposal”) and a proposal (the “Delaware Conversion Proposal”) to approve the reincorporation of TuHURA from Nevada to Delaware (the “Delaware Conversion”). The approval of the Delaware Conversion Proposal is not a condition to the completion of the Mergers, but is being presented to TuHURA stockholders for approval at the TuHURA special meeting. The TuHURA board of directors anticipates that the Delaware Conversion will occur upon the earlier of (i) such time as determined by the TuHURA board of directors following the completion of the Mergers or (ii) in the event that the Mergers are not consummated or the Merger Agreement is terminated in accordance with its terms, at such time as determined by the TUHURA board of directors. Kineta stockholders are cordially invited to attend a special meeting of stockholders of Kineta to be held on [●], virtually via the Internet at [●], at [●], Eastern Time, including any adjournments or postponements thereof (the “Kineta special meeting”, together with the TuHURA special meeting, the “special meetings”) to consider and vote on the proposal to adopt the Merger Agreement (the “Merger Agreement Proposal”) and the proposal to approve, on a non-binding advisory basis, specific compensatory arrangements between Kineta and its named executive officers relating to the Mergers (the “Compensation Proposal”).
If the Mergers are consummated, Kineta stockholders will be entitled to receive , without interest, (x) the number of validly issued, fully paid and non-assessable shares of common stock, $0.001 par value per share, of TuHURA (“TuHURA Common Stock”) (rounded down to the nearest whole share subject to the payment of any cash in lieu of fractional shares as set forth in the Merger Agreement) equal to (i) the Initial Per Share Stock Consideration plus (ii) the Delayed Per Share Stock Consideration and (y) plus an amount in cash equal to the (i) Per Share Cash Consideration plus (ii) the Disposed Asset Payment Right all as described in the Merger Agreement (collectively, the Initial Per Share Stock Consideration, the Delayed Per Share Stock Consideration, the Per Share Cash Consideration and the Disposed Asset Payment Right, the “Merger Consideration”).
The Merger Consideration will not be adjusted in the event of any change in the price of either TuHURA Common Stock or shares of common stock of Kineta, par value $0.001 per share, (the “Kineta Common Stock”) that occurs during the period following the signing of the Merger Agreement and closing of the Mergers as the share value of TuHURA Common Stock included in the calculation of Initial Per Share Stock Consideration and the Delayed Per Share Stock Consideration is fixed at $5.7528 (the “TuHURA Share Value”) pursuant to the terms of the Merger Agreement. However, although the number of shares of TuHURA Common Stock issuable in the transaction will not fluctuate with market prices given that the TuHURA Share Value is fixed, the market value of the Merger Consideration will fluctuate with the price of TuHURA Common Stock given TuHURA Common Stock is traded on the Nasdaq Capital Market.
As part of the Merger Consideration, Kineta stockholders will be entitled to payments of legacy assets not related to the VISTA program through the Disposed Asset Payment Right as Kineta has also entered into three separate agreements to sell its assets as permitted under the Merger Agreement. Kineta has entered into an Asset Purchase Agreement dated February 4, 2025, as may be amended from time to time (the “HCRX Asset Purchase